1. Introduction
In 1950s, methotrexate (MTX) was introduced as a systemic immunosuppressive agent for treating various dermatological and rheumatological disorders (1, 2). It is an antifolate that binds to an enzyme, dihydrofolate reductase, which catalyzes the conversion of dihydrofolate to tetrahydrofolate, the active form of folic acid. Tetrahydrofolate is required for formation of one carbon metabolites during DNA synthesis, thus ultimately leading to the inhibition of DNA synthesis (3). Methotrexate can be safely used for the treatment of psoriasis, systemic lupus erythematosus, vasculitis, and many other connective tissue disorders, etc. (4, 5). Accidental toxicity with methotrexate occurs in 3 - 10% of patients receiving methotrexate medication in high doses (6). Here, we report one such case of acute toxicity with methotrexate in a patient with psoriasis.
2. Case Presentation
A 75-year-old woman was brought to the emergency department of our tertiary care center with complaints of oral intolerance to spicy food and raw areas in the mouth and all over the body with profuse bleeding from the back for one month with intermittent episodes of fever. She was a known case of type 2 diabetes mellitus from one year ago and was on regular medications. However, she was unable to offer a detailed history due to her poor general condition. There was no history of mucocutaneous ulcerations in the past in the patient or in other members of her family.
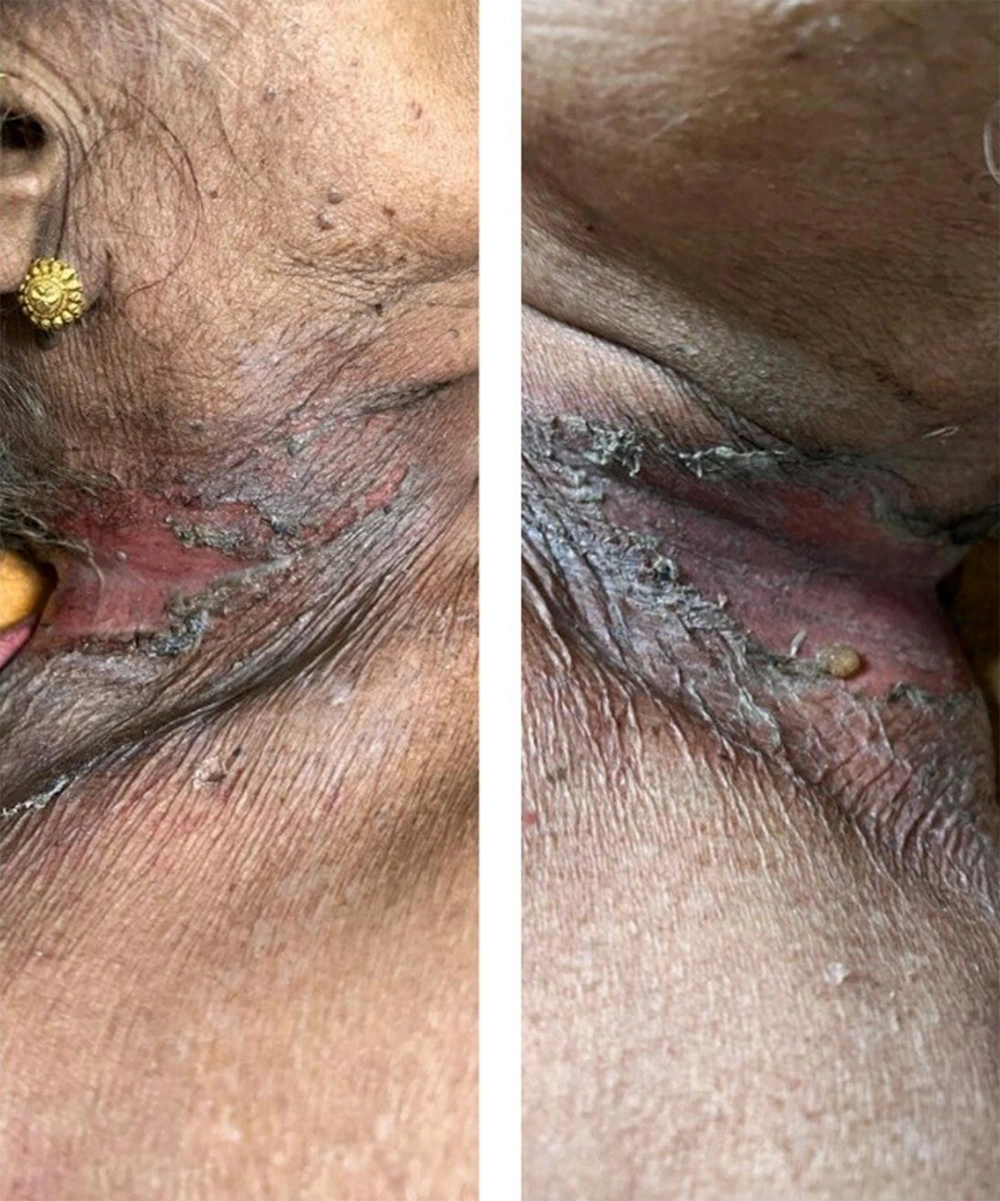
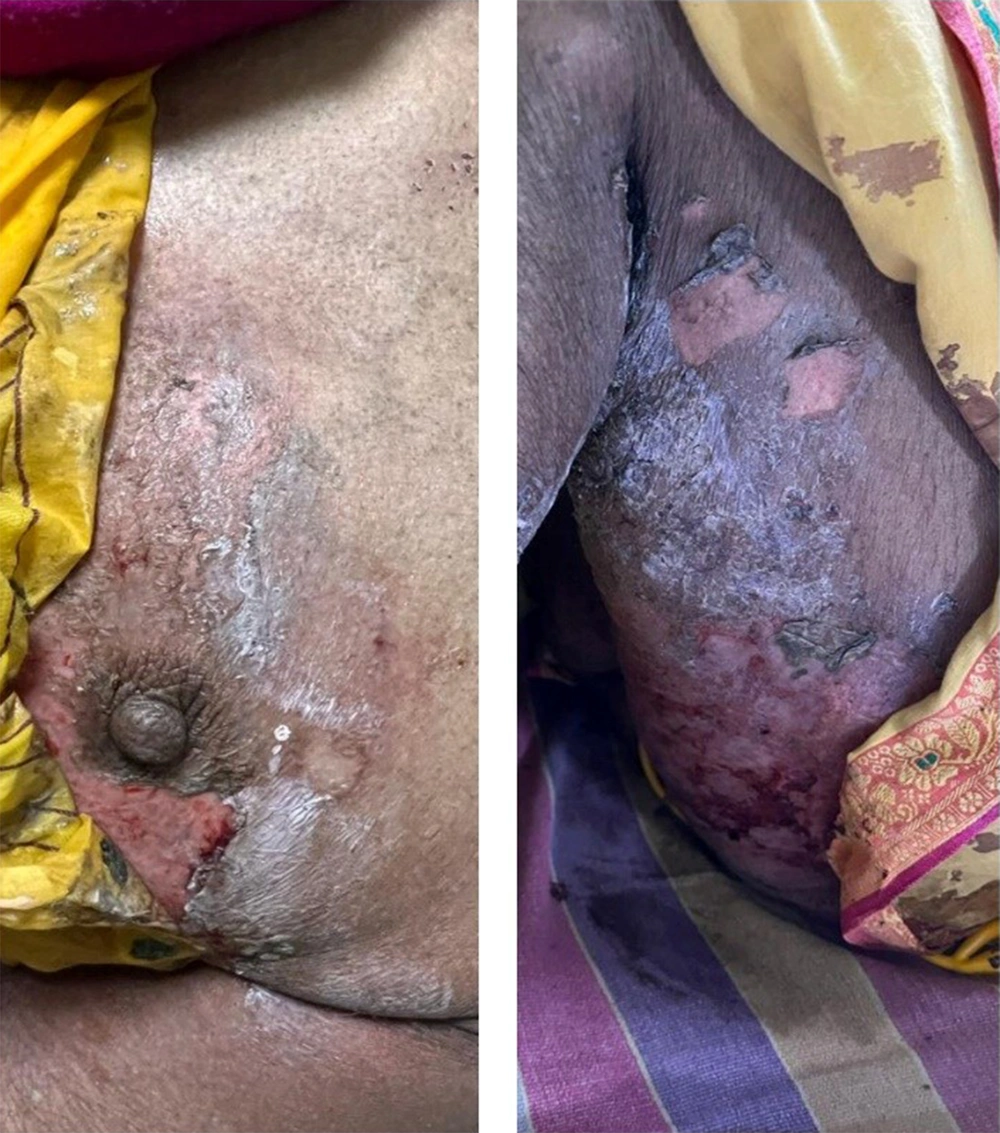
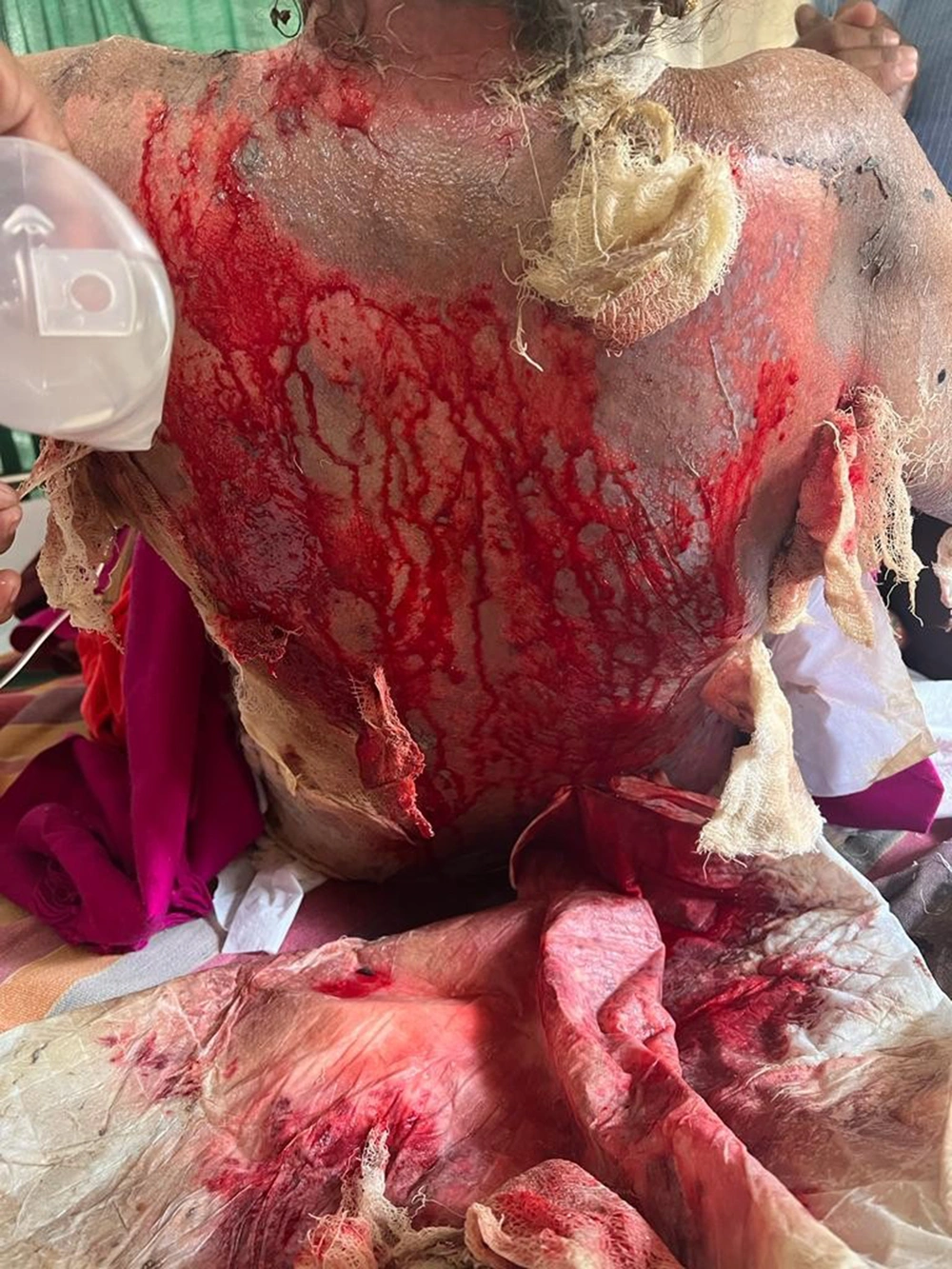
On dermatological examination, she had multiple erosions over the neck (Figure 1), axillae, mammary areas (Figure 2), lower extremities (Figure 2), and back with profuse spontaneous bleeding (Figure 3). Oral mucosa showed multiple whitish plaques and erosions over the tongue, buccal mucosa, and palate. On tangential pressure, shearing of the epidermis was noticed. Scalp and face were spared. Palms, soles, and nails were normal. There was no bleeding from other orifices. Systemic examination was normal.
Clinical differential diagnoses were autoimmune vesiculobullous disorders (pemphigus Vulgaris and paraneoplastic pemphigus) and Stevens-Johnson syndrome-toxic epidermal necrolysis (SJS/TEN). Laboratory evaluation showed anemia with hemoglobin (9 gm/dL), decreased total leukocyte count (500/mm3), transaminitis with aspartate aminotransferase (AST) 99 units/L, alanine aminotransferase (ALT) 72 units/L, positive C-reactive protein (CRP). The rest of the investigations were within normal limits. Skin biopsy showed an ulcerated epidermis with focal areas of parakeratosis. Bacterial colonies were noted in the stratum corneum. Artifactual separation of epidermis and dermis was seen. Dermis showed edema, and mild perivascular inflammatory infiltrate, with areas of hemorrhage. Direct immunofluorescence studies did not show any positivity for antisera specific against IgG, IgM, and C3.
Subsequent detailed interrogation of relatives and scrutiny of past medical records revealed that the patient had a history of generalized pus-filled lesions (with onset from extremities) diagnosed as pustular psoriasis clinically by a private practitioner and started on cap acitretin 25 mg at night daily and tablet of methotrexate 5 mg once a week for four months. Since last three weeks prior to presentation, she had been taking tablet of methotrexate 5 mg two times per day. There was no other contributory drug history. Hence correlation of clinical findings with the history led to the diagnosis of acute methotrexate toxicity. Immediately methotrexate was stopped, and she was started on supportive treatment with IV fluids, IV antacids, IV antibiotics such as vancomycin, antibiotic-impregnated gauzes for erosions, oral care, and injection of paracetamol for fever. Leucovorin (50 mg/5 mL, 1.5 mL in 500 mL normal saline) was administered every eight hours for a total of 10 doses, along with injection of filgrastim (colony-stimulating factor) 300 mcg subcutaneously once a day for three days for neutropenia. Urine alkalinization was carried out with sodium bicarbonate, and the patient was stringently monitored in terms of vital parameters. Her total leukocyte count increased to 1,500 followed by improvement of mucocutaneous lesions.
3. Discussion
Methotrexate toxicity is characterized by nausea, diarrhea, mucocutaneous ulcerations, pancytopenia, or gastrointestinal bleeding (7-9). Methotrexate, when given in high doses (> 1 g/m2), can lead to kidney injury, whereas renal dysfunction can cause methotrexate toxicity due to a decrease in renal clearance (10-13). However, mucocutaneous ulceration can be an early clinical sign of imminent systemic toxicity, and isolated mucosal or cutaneous lesions may sometimes be the only presentation seen in 3 - 10% of patients (14-16). In the event a patient takes an overdose of methotrexate or takes it before the next scheduled weekly day, the proliferation of escaped cells will not occur, and ultimately, more than requisite cells will get affected, thereby resulting in erosions and ulcerations as a manifestation of methotrexate toxicity (17). The clue which assists in early recognition is the burning sensation and pain in psoriatic plaques, which is out of proportion to the clinical appearance of lesions (18, 19).
Methotrexate-induced side effects can be due to dose-dependent or idiosyncratic mechanisms. Incorrect intake of drug and various drug interactions are contributory factors (8). These drugs include proton-pump inhibitors, trimethoprim/sulfamethoxazole, doxycycline, non-steroidal anti-inflammatory drugs (NSAIDs), and salicylates that decrease protein binding or reduce renal clearance, as well as excessive alcohol consumption can play an important role in Methotrexate toxicity (17). In our case, incorrect intake of drug was identified as the causative factor, which was different than usual presentations of methotrexate toxicity because of resemblance of lesions with autoimmune vesiculobullous disorders or severe drug reactions like (SJS/TEN); however, histopathology and direct immunofluorescence studies ruled out both differential diagnoses. Although she was a diagnosed case of psoriasis, there was no clinical or histopathological evidence of the same at presentation, which might be attributed to the ulceration of the pre-existing psoriatic lesions.
Withdrawal of methotrexate and administration of intravenous folinic acid (leucovorin) as early as possible after exposure is an essential initial treatment that should not be delayed at any cost (2). Supportive treatment with IV fluids, IV antacids, IV antibiotics, antibiotic-impregnated gauzes for erosions, oral care, colony-stimulating factor (filgrastim) if severe neutropenia, urine alkalization with bicarbonate infusion (if indicated) should be started simultaneously. Improvement in clinical manifestations can occur as early as ten days after discontinuation of methotrexate (7). The patient should be carefully monitored for vital signs, as well as any signs of infections or bleeding and abnormalities in laboratory parameters to assess renal and hepatic function.
3.1. Conclusions
Acute methotrexate toxicity can rarely present with extensive mucocutaneous ulcerations mimicking autoimmune vesiculobullous disorders or (SJS/TEN). In such challenging and potentially life-threatening scenarios, a low threshold of suspicion supported by astute history-taking and detailed evaluation is essential. Therefore, timely diagnosis and prompt treatment (preferably within an intensive care setup) and regular follow-up of such patients can avert complications and improve outcomes.