1. Introduction
Recently topical 3% diclofenac is commonly used to treat actinic keratosis (AK) with good results (1). It may also have a potential effect on hair growth (2). Authors present three cases of hair growth on the scalp after topical diclofenac treatment. To the authors’ best knowledge, these are the first reported cases in the literature.
2. Case Presentation
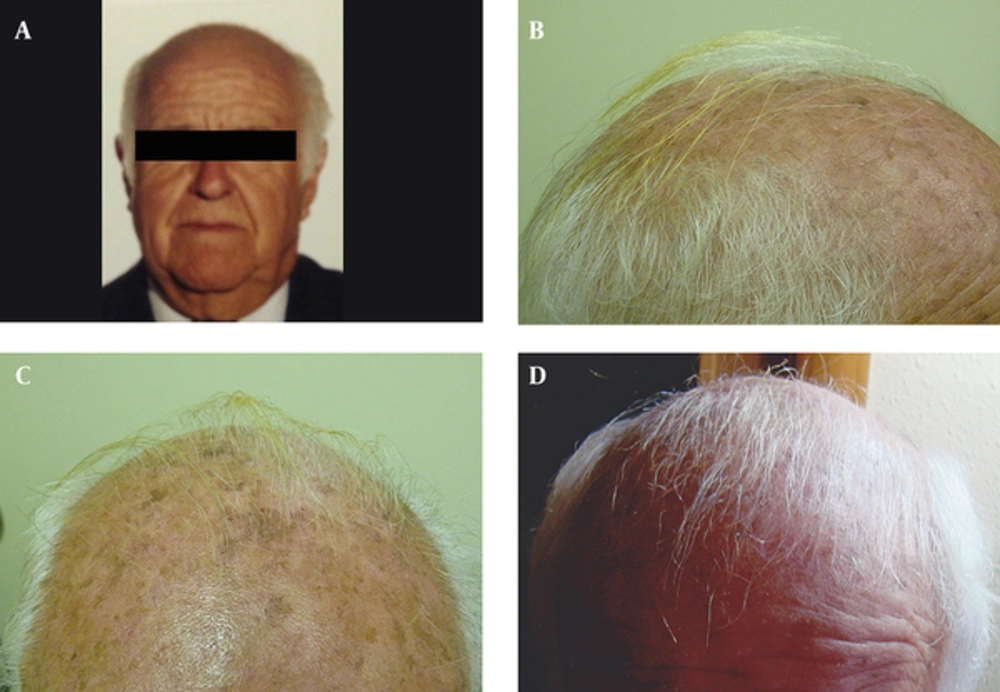
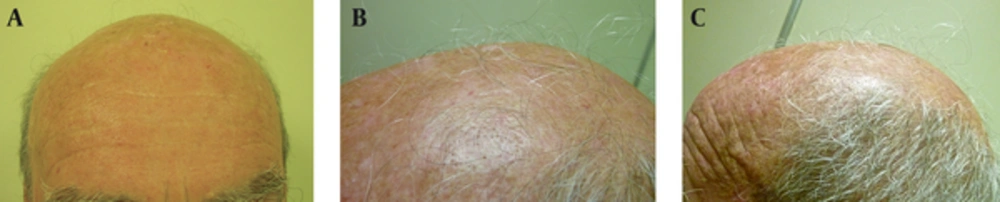
Three male patients were visited in the dermatology department because of scalp AK, and received treatment with topical 3% diclofenac gel-based drug compound, once nightly for four to six months. One gram of the gel was used on a 10 × 10 cm area. All patients were in their seventies (73, 77 and 79 years old) and had also concomitant androgenetic alopecia for more than 15 years. During the follow-up visits after four and six months of starting the treatment, all patients and their families described terminal hair growth on non-prior haired areas of the scalp. This finding was also observed by the authors (Figures 1 and 2). There were neither relevant previous interventions nor concomitant medications that could interfere in the outcome. Patient 1 was followed-up for four years because of his AK, and showed continuous hair growth over the span of this period (Figure 1). Treatment was well tolerated in all cases and no adverse events were observed.
A, Photo provided by the patient, taken two years before the treatment, showing scalp with absence of hair; B and C, Evidence of terminal hair growth over non-prior haired biparietal area, after a six-month treatment with topical diclofenac 3% gel-based drug compound; D, After four years follow-up, the patient showed abundant white hair regrowth.
3. Discussion
Androgenetic alopecia (AGA) is an alteration of hair growth and/or a premature aging of the pilosebaceous unit with a multifactorial etiology. The contribution of a genetic and metabolic androgen imbalance in AGA may be locally exacerbated and amplified at the pilosebaceous unit level by pro-inflammatory mediators and cytokines. Some authors call the implication of various activators of inflammation in the etiology of AGA as micro-inflammation (3). Perifollicular micro-inflammation may account for some cases of male pattern AGA, which do not respond to topical minoxidil (3).
The inflammation pathway of the arachidonic acid (AA) is clearly identified, and several anti-inflammatory drugs that inhibit this aspect of inflammation are developed and clinically evaluated. The AA is metabolized through two families of enzymes, generating either prostaglandins (PGs) (through the activity of prostaglandin-endoperoxide synthase 1 and 2, also named cyclooxygenase 1 and 2 (COX-1 and COX-2)) or leukotrienes and hydroxyeicosatetraenoic acid (through the action of lipoxygenases). These mediators have an implication in vasodilatation, nonspecific chemotaxis, complement cascade activation, inflammatory cells infiltration and thickening of the dermal sheath of the hair follicle, which may lead to micro-inflammation and perifollicular fibrosis observed in AGA (3). Matrix metalloproteinases (MMPs) may play an active role in the perifollicular fibrosis that follows inflammation.
COX-2 has a key function in the prostaglandins pathway, converting AA to PGH2, from which PGD2, PGE2, PGF2α, prostacyclin and thromboxane A2 are produced by specific synthase enzymes. Recent publications demonstrate that PGs are implicated in hair growth regulation. In the hair follicle, increased PGs levels through over-expression of COX-2 favor alopecia (4). Also, it is found that mice overexpressing the COX-2 enzyme develop alopecia (5). Frequently, as it occurs in other organs, different PGs have opposing biological effects. In the case of the hair follicle, PGD2 inhibits hair growth, whereas PGE2 and PGF2α enhance it (4, 6). A recent study found that PGD2 levels significantly increased in bald scalp compared with haired scalp of patients with AGA (7).
Treatments for AGA used in daily clinical practice also support PGs’ implication in hair follicle function. Minoxidil, a reference treatment to stimulate hair regrowth, increases PGE2. Bimatoprost and latanoprost, PGF2α analogs, are recently used successfully to increase the length of eyelashes (6, 8).
Diclofenac 3% gel is a non-steroidal anti-inflammatory drug that inhibits COX-1 and COX-2 enzymes, resulting in decreased production of PGs and MMPs. The effectiveness of diclofenac 3% gel to treat AK is already demonstrated in placebo-controlled clinical trials and is described elsewhere (1, 9). Its potential effect on hair regrowth in alopecia areata is reported (10).
Since the AA pathway is involved early in the inflammatory process, acting on this aspect of inflammation might be useful to decrease micro-inflammation and benefit against AGA. Increased inflammation may potentially upregulate greater COX-2 expression and thereby make COX inhibition more effective. Authors hypothesize that the inhibition of the COX-2 by diclofenac, might act over the inflammatory mechanism and the PGs misbalance (↑PGD2, ↓PGE2, ↓PGF2α) observed in the AGA.
In the current cases, diagnostic methods of hair growth included patients’ descriptions, authors’ evaluations and clinical images; however as a potential drawback, no laboratory testing or specific imaging surveys were conducted. Another limitation of the study was the limited number of patients; therefore, the findings need to be confirmed in a larger study.
3.1. Conclusions
Various hypotheses are proposed to explain the causes of AGA, including androgens, prostaglandins misbalance and perifollicular micro-inflammation. In the hair follicle, over-expression of COX-2 enzyme favours alopecia. Also PGD2 levels were found significantly increased in bald scalp compared with haired scalp of patients with AGA. The authors present three cases of patients with AK and concomitant androgenetic alopecia treated with topical 3% diclofenac gel, who showed terminal hair growth on non-prior haired areas of the scalp. Authors believe that this finding will be interesting for dermatologists and may open new research lines, however further investigations are needed to elucidate the mechanisms of micro-inflammation and PGs regulation in AGA to offer improved treatment options to patients.