1. Context
Cell therapy is the transplantation of autologous or allogeneic cells through local delivery or systemic administration to restore the viability or function of deficient tissues. Stem cells are the best choice for cell therapy because of their high potential for self-renewal, differentiation, and plasticity (1). Adult tissues contain stem and or progenitor cells that are capable of producing the progeny of other stem cells (self-renewal) or asymmetrically dividing into other stem cells (2).
The skin is the largest organ of the human body, making up to 15% of body weight. The skin is a natural target for stem cell research because of its large size and ease of accessibility. Subcutaneous tissue is a source of stromal stem cells having the capacity for multipotential differentiation (3). Dermis and adipose tissue are promising alternatives to bone-marrow-derived stem cell therapies. The present study provides a broad overview of the current knowledge about skin multipotent mesenchymal cells (MSCs) and their application for cell therapy (4).
1.1. The Nature of Stem Cells
Stem cells are unspecialized cells with 2 important properties that distinguish them from other cells in the body. First, they can restore their numbers over long periods. Second, after receiving certain chemical signals, they can differentiate into specialized cells with specific functions such as heart, nerve, or skin cells (5). Research is now being carried out on skin stem cells to distinguish their characteristics and potential for curing disease.
1.2. Stem Cell Characteristics
Stem cells represent undifferentiated cells that can proliferate without differentiation over a long period. They can also give rise to mature cell types that have characteristic shapes and specialized functions. The most important property of a stem cell is its plasticity. The term “transdifferentiation” often used synonymously with “plasticity” and refers to the ability of adult stem cells to differentiate into tissues other than the ones from which they originated, for example, differentiation of adult bone-marrow-derived MSCs into multiple skin cell types.
The concept of transdifferentiation is in doubt; however, according to some researchers the actual incidence of transdifferentiation in vivo is exceptionally rare (6). They believe that other routes such as cell fusion actually occurs (7), as when bone-marrow-derived stem cells regenerate liver cells by fusion with pre-existing hepatocytes.
The findings of in vivo and in vitro studies of cutaneous MSCs contradict (8). Reports suggest that multiple mechanisms, including transdifferentiation, cell fusion, and production of growth factors may exist that allow MSCs to exhibit multipotentency (9). Stem cell migration, which is key to their development and regeneration, is regulated by the SDF-1/CXCR4 axis (stromal-derived factor-1/chemokine [C-X-C motif] receptor-4) (10-12). The CXCR4 receptor has been described in many types of tissue-specific stem cells, including nervous tissue, skeletal muscle, heart, liver, endothelium, nephron tubules, and pigment cells of the retina (13).
1.3. Types of Skin Stem Cells
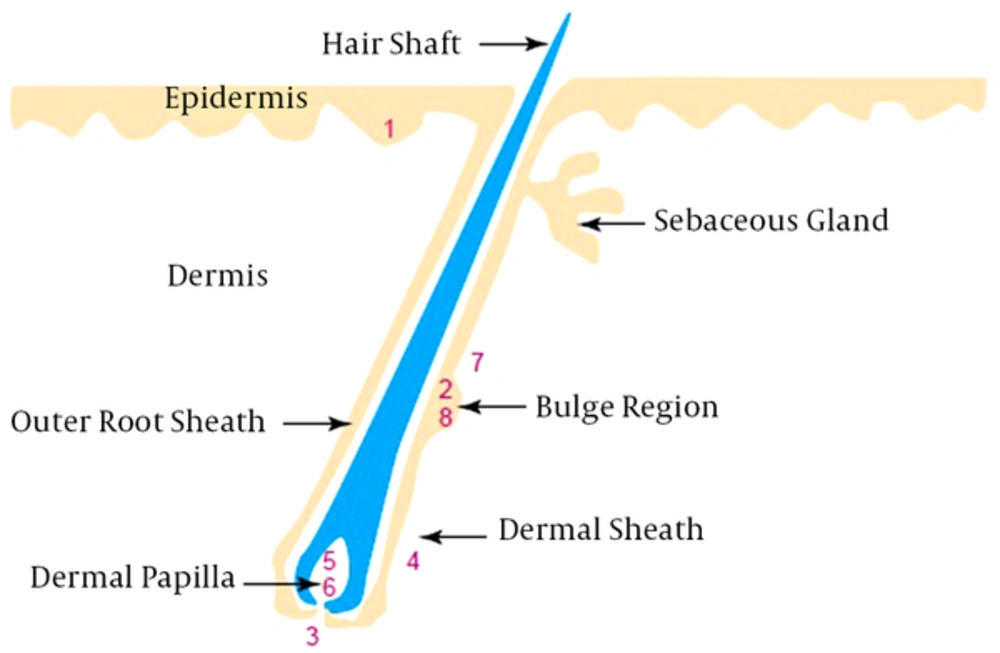
Skin stem cells are located in the epidermis, dermis, and hair follicles as epidermal, follicle multipotent, mesenchymal, dermal sheath, neural crest, hematopoietic, endothelial, and melanocyte stem cells (Figure 1) (5). Epidermal stem cells (ESCs), follicle multipotent stem cells, and melanocyte stem cells are located in the epidermis. ESCs are derivative cell types that include transient amplifying cells and terminal-differentiated epidermal cells (14, 15). Follicle multipotent and melanocyte stem cells located in the hair follicle bulge region can derive a spectrum of cells, including hair follicle epithelium (including the outer root sheath, inner root sheath, and hair shaft), sebaceous gland cells, epidermal cells, and melanocyte cells (16).
Mesenchymal and endothelial stem cells in the dermis can derive mesodermal and endothelial cells, respectively (17). Neural-crest and hematopoietic stem cells from hair follicle dermal papilla derive all neural cell types and blood cell lineages (18). Dermal-sheath stem cells located in the hair follicle dermal sheath derive dermal papilla cells and wound-healing fibroblasts (19).
2. Evidence Acquisition
There are many therapeutic advantages to the exploitation of skin cell plasticity. Skin cells as a stem cell source are abundant, easy to access, have a high self-renewal ability, and can possibly be used for autologous transplantation for practical therapeutic treatment. The unique immunological profile of the hair follicle makes it an ideal source for autologous or allogeneic applications. There is evidence that the hair follicle locally produces potent immune suppressants such as TGF-β1 (transforming growth factor beta 1), IL-10 (interleukin 10), and α-MSH (α-melanocyte-stimulating hormone). The absence of classic MHC (major histocompatibility complex) class I expression has also been observed (20). Initial research on cutaneous MSCs was carried out by Toma et al. (21). This study was the first to isolate multipotent adult stem cells from the dermis. Researchers from Pennsylvania and California later isolated and cultured stem cells of mice hair follicles. Clinical applications are now rapidly expanding, but much more work must be done.
3. Results
3.1. Skin Cell Therapy in Wound Healing
Wound healing is a complex process that requires ECM, growth factors, and cells. MSCs can mediate each phase of wound-healing; i.e., inflammatory, proliferative, and remodeling. Among the main sources of cells that might be used for repair and regeneration of injured skin are adult stem cells, ESCs, and induced pluripotent stem cells. Adult human epidermal stem cells in the epidermis and the hair follicle are also used for transplantation in deep burn wounds, particularly when combined with fibrin matrices to facilitate transplantation of epidermal stem cells (22). Badiavas et al. (23) conducted clinical studies using fresh and culture autologous bone marrow MSCs for the treatment of chronic wounds. They made significant progress in the regeneration of the dermis for wound healing through transplantation of bone marrow MSCs. Adipose tissue stem cells injected into wounds and diabetic foot ulcers have been found to reduce scar formation and healing (24). Cell suspension of adipose-derived stem cells was also utilized with fibrin glue injected into the wound to cure complex perianal fistulas in a phase II clinical trial by Anterogen Co., Ltd. (25).
3.2. Skin Cell Therapy and Hematopoietic System
Skin stem cells, especially MSC-like cells obtained from the dermis, were found to act as an alternative source of marrow stromal cells to repopulate the microenvironments of the mouse hematopoietic system, but these cells could not differentiate into hematopoietic cells (26). Hair follicle end bulbs have been shown to produce hematopoietic cells of erythroid and myeloid lineages in lethally irradiated mice (27).
3.3. Skin Cell Therapy in the Nervous System
Neural stem cells obtained from the skin could provide a novel therapeutic strategy and accessible source of treatment for nervous system disease. Skin stem cells such as epidermal, dermal multipotent MSCs, skin-derived precursors (SKPs), and dermal fibroblasts have the potential to differentiate into neurons and glial cells under proper conditions. They have shown the ability to repair spinal cord injury after transplantation (28). Studies have shown that SKP cells can passage for up to 12 months. It has been reported that EGF, basic fibroblast growth factor (bFGF), and TGFβ can increase differentiation potential (5).
3.4. Current Treatment Strategies in Wound Healing
Skin grafting has been a challenging task for researchers and tissue engineers since its introduction in 1871 by Reverdin (29). Innovative tissue engineering approaches based on scaffolds and clinical grade stem cells are attractive alternatives available as skin substitutes. Despite being clinically useful, skin grafts have limitations such as pain, scarring, slow healing, infection, immune rejection in allogenic skin grafts, and unavailability of the donor site in circumstances of extensive skin loss. For these reasons, scientists have worked to find skin substitutes to replace skin defects. These materials are used to cover skin defects (30).
To date, a number of biological and synthetic skin substitutes have been made commercially available. These products have been classified into 3 categories. The first is based on their origin in the skin layers (31) as epidermal, dermal, and dermal-epidermal skin substitutes (32). The second category is durability and comprise temporary and permanent skin substitutes (33). The third category is compatibility of commercially-available substitutes as biological (autologous basal keratinocytes), synthetic (containing an inner layer of nylon mesh and an outer layer of silastic), and bio-synthetic that use a scaffold-based bilayer skin substitute containing hyaluronan with autologous fibroblasts and an outer silicone membrane (34).
Clinical skin replacements and grafts are in high demand for the treatment of skin injuries: they represent approximately 50% of tissue engineering and regenerative medicine market revenue (35). While engineered skin substitutes represent a significant improvement in wound healing, especially for burns and diabetic foot ulcers, their use is not routine because of high price, limited effectiveness, and their inability to renew skin appendages (36).
4. Conclusions
Skin stem cells have the potential to function as an easily accessible autologous source of future stem cell transplantation. Different therapeutic applications exist for these cells. The most immediate effect can be expected in the area of wound healing. Further comprehensive research is needed to understand the cellular and molecular events involved in the development, repair, and regeneration of the skin and its associated structures.
Skin stem cells have therapeutic potential for a variety of diseases coupled with their ability to differentiate into many cell types, ease of accessibility, and a unique immunological profile. One challenge for future research is to facilitate wound healing of chronic wounds. Skin stem cells provide hope for this outcome; however, further study is needed to determine the fate of transplanted cells and the number of cells required to show precise effects. The availability of skin-derived progenitors provides opportunities to address limitations of other therapeutic methods without the risk of immune rejection of a transplant. Apparently, skin stem cells are suitable candidates for use as universal donors in cell-based therapies and regenerative medicine.