1. Background
Alopecia is one of the common conditions for which patients consult dermatologists. It is broadly classified as non-cicatricial causes like patterned hair loss, telogen effluvium, alopecia areata, etc., and cicatricial causes like discoid lupus erythematosus, and lichen planus pigmentosus, etc. This differentiation can often be difficult, and a trichoscopy would help with a non-invasive and rapid diagnosis in such situations. The need for an accurate diagnosis of various patterns of alopecia cannot be overstated because of the different treatments. In this era, where rapid, non-invasive diagnostic procedures are at the forefront, the importance of trichoscopy is invaluable. Trichoscopy, a rapidly evolving diagnostic modality, is a new method to diagnose hair loss using dermoscopy of the hair, scalp, eyebrows, and eyelashes to visualize and measure hair at high magnification (1). Lacarrubba et al. first described videodermoscopic features of alopecia areata (2). Olszewska et al. first used video-dermoscopy to evaluate disease severity in androgenic alopecia and to monitor treatment efficacy (1). It helps to monitor the response to treatment (3). In a busy OPD, trichoscopy helps to have an accurate diagnosis, thus, avoiding undue delay in starting the treatment, thus making the need to know about trichoscopy of various conditions imperative. This cross-sectional study was conducted to observe the various patterns of alopecia and evaluate its usefulness in aiding clinical diagnosis. Multiple studies describe the classical trichoscopy features of various types of alopecia, and we tried to look for novel, subtle dermoscopic findings among patients with skin of color and compare the dermoscopic features across all patterns of scarring and non-scarring alopecia (4).
2. Objectives
To document the dermoscopic findings of various patterns of alopecia and the utility of trichoscopy in doubtful cases of clinical diagnosis.
3. Methods
After approval of the institutional ethics committee, a convenient sampling of 100 patients with alopecia who were willing to give informed consent were enrolled in the study. A detailed history of the patients, including family, medication, personal history, habits, and tics, was recorded. The diagnosis was made based on history and clinical examination findings. Only in case of ambiguity of the clinical diagnosis was a biopsy performed. Relevant blood investigations like complete blood count, KOH mount (potassium hydroxide), fungal culture, serum vitamin B12 levels, serum vitamin D levels, thyroid function tests, serum estrogen, testosterone, follicle stimulating hormone (FSH), luteinising hormone (LH) were done in indicated cases. Also, a biopsy was performed in all cases of cicatricial alopecia. Clinical photographs were taken. Trichoscopy was performed using illuco IDS- 1100 with 10x magnification. female pattern hair loss (FPHL) was clinically suspected in cases of widening of midline parting without the involvement of occiput. In diffuse alopecia patients, trichoscopic images were taken at 5 cm on both sides of the midline and 3 cm from the anterior and posterior hairlines. All dermoscopic findings were recorded. Data were analyzed using SPSS 22 version software. The correlation between clinical and dermoscopic diagnosis was assessed using kappa statistics. A P-value (probability that the result is true) of < 0.05 was considered statistically significant.
4. Results
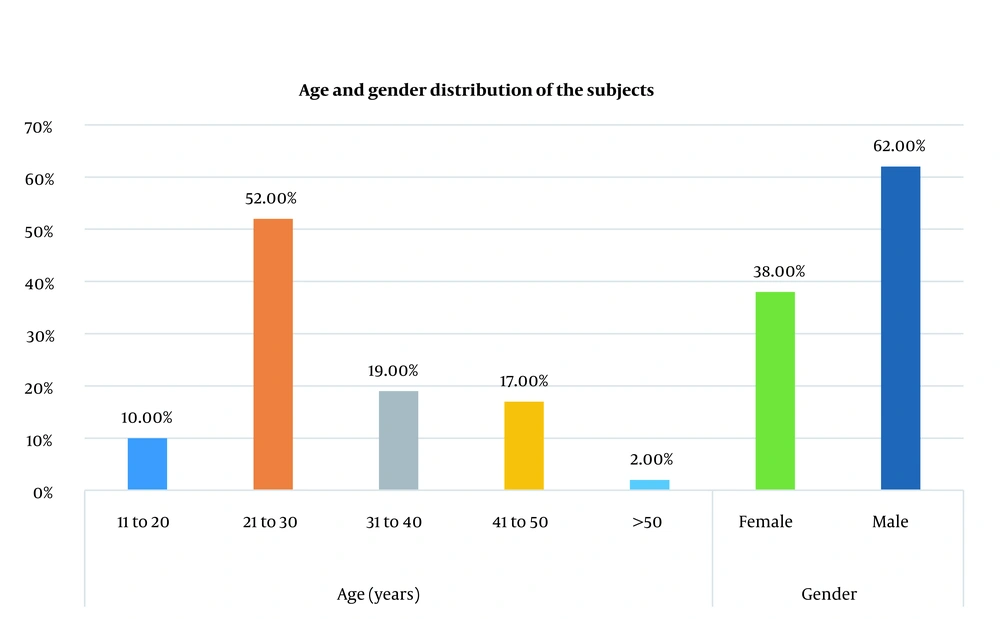
One hundred alopecia subjects were enrolled in this study. Sixty-two were male, and 38 were female (Figure 1). Most subjects were between the ages of 21 and 30 (52%) of the sample size. According to the study, our subjects had a mean age of 28.86 ± 9.852 years. Ninety-three subjects had nonscarring alopecia, and 7 had scarring alopecia (discoid lupus erythematosus).
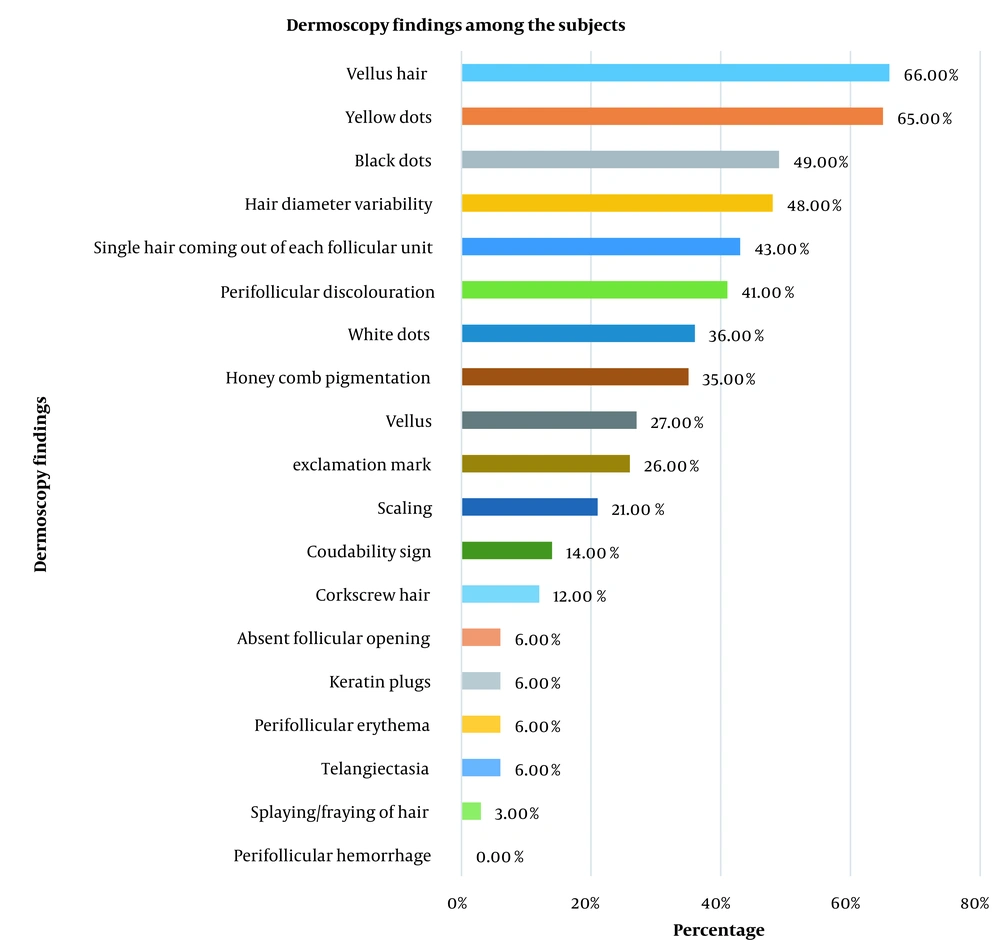
Among 42 subjects with androgenetic alopecia (AGA), on trichoscopy, vellus hair was the most common finding (90.48%), followed by hair diameter variability and yellow dots (83.33%), 30 (71.43%) had single hair coming off the follicular unit, 6 (14.3%) had scaled, 38 (90.5%) had vellus hair, 27 (64.3%) had honeycomb pigmentation, 28 (66.67%) had perifollicular brown pigmentation, 8 (19.05%) had black dots, and 8 (19.05%) had white dots.
Out of 10 subjects who had FPHL on clinical examination, all had vellus hair, 9 (90%) had hair diameter variability, 7 (70%) had perifollicular pigmentation, 6 (60%) had honeycomb pigmentation, 6 (60%) had black dots, 4 (40%) had scaled, and 4 (40%) had single hair coming out of each follicle unit, and 1 (10%) had white dots.
Out of 30 clinically diagnosed as alopecia areata, 26 (86.67%) had vellus hair, and 26 (86.67%) had exclamation mark hair. A total of 25 (83.3%) had black dots and white dots each. 23 (76.67%) had yellow dots. 18 (60%) had vellus hair. 13 (42.33%) had coudability signs, and 4 (13.33%) had variability in hair shaft diameter.
Out of 8 subjects clinically diagnosed with tinea capitis, all had corkscrew hair and black dots as the commonest finding, followed by scaling (90%). Other findings were yellow dots (25%), perifollicular discoloration (12.5%), and white dots (12.5%).
Out of 6 biopsy-proven cases of discoid lupus erythematosus, all had classical features like absent follicular opening, scaling, perifollicular pigmentation, keratin plugs, and telangiectasia.
Among the 3 cases diagnosed clinically as trichotillomania, all had hair splaying with other features like scaling, yellow dots, and black dots. We could not find classical features like perifollicular hemorrhages.
We enrolled one patient who developed alopecia following external beam radiotherapy for thyroid carcinoma and showed features like yellow dots, scaling, and black and white dots. However, a biopsy could not be performed due to a loss of follow-up.
In the study, among all types of alopecia, common findings on trichoscopy were vellus hair (66%) followed by yellow dots (65%) (Figure 2).
Out of 15 patients with discordant clinical and dermoscopic findings, further workup among these subjects showed that the dermoscopic diagnosis was accurate in 7 subjects (Table 1).
| Clinical Diagnosis | Number of Patients | Dermoscopic Diagnosis | Investigations | Final Diagnosis |
|---|---|---|---|---|
| Androgenetic alopecia | 9 | Alopecia | Hormonal analysis, USG (abdomen and pelvis), scalp biopsy | 5 had androgenetic alopecia; 4 had alopecia areata |
| Alopecia areata | 5 | Androgenetic alopecia | Scalp biopsy, hormonal analysis, USG (abdomen and pelvis) | 2 had alopecia areata; (3 refused to biopsy) |
| Tinea capitis | 1 | Alopecia areata | KOH mount and culture | Tinea capitis |
Comparison of Dermoscopic Diagnosis and Clinical Diagnosis
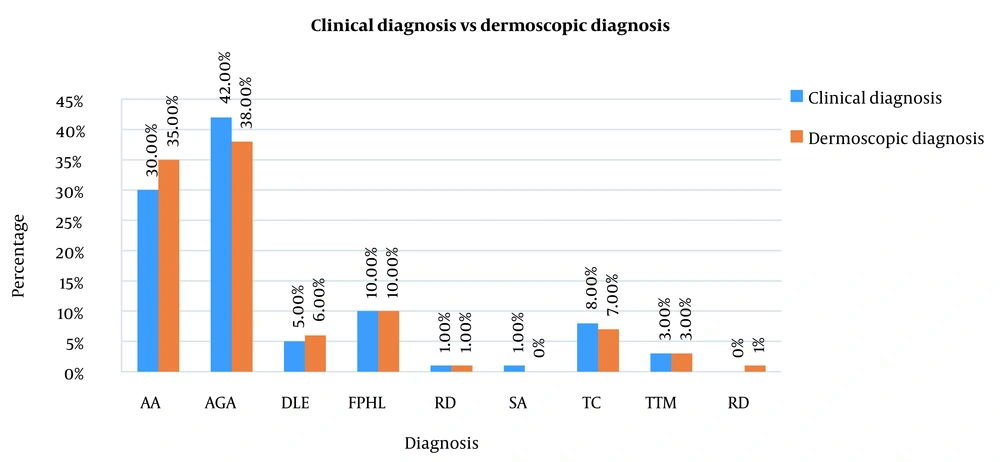
There was a significant association between clinical diagnosis and dermoscopic diagnosis. Kappa agreement was 0.776 (i.e., substantial agreement) between clinical and dermoscopic diagnoses (Tables 2, 3 and Figure 3).
| Dermoscopic Diagnosis | Clinical Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|
| AA | AGA | DLE | FPHL | RD | SA | TC | TTM | |
| AA | 25 (83.3) | 8 (19.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) |
| AGA | 4 (13.3) | 34 (81.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| DLE | 0 (0.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| FPHL | 1 (3.3) | 0 (0.0) | 0 (0.0) | 9 (90.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| RD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TC | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (87.5) | 0 (0.0) |
| TTM | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) |
| Value | SE | Approx. T | P Value | |
|---|---|---|---|---|
| Measure of agreement (kappa) | 0.776 | 0.052 | 13.649 | < 0.001 a |
| N of valid cases | 100 |
Symmetric Measures
5. Discussion
Alopecia which significantly affects the psychology of a person is also one of the most common problems that bring patients to a dermatologist’s office. Alopecia can be patterned or diffuse and cicatricial or non-cicatricial. In this study, we recorded the most common dermoscopic features of alopecia and compared the dermoscopic diagnosis with the clinical diagnosis.
In a study conducted by Inui et al., scalp trichoscopy in 50 AGA Asian men showed that all the subjects had hair diameter variability, and peripilar sign was seen in 66% of subjects (5). These findings were similar to our study. These observations were also similar to those seen in studies conducted by Ummiti et al. (6).
Although yellow dots are considered classically seen in alopecia areata, in a study conducted by Chiramel et al. in evaluating trichoscopic features for AGA, all the subjects had yellow dots, unlike ours, where 83.33% had yellow dots (7). But their study showed that only 40.9% of subjects had vellus, while in our study, vellus hair was the most common finding among AGA subjects. Another study by Ummiti et al. concluded that yellow dots was found in 92.4% of males with AGA (6).
Female pattern hair loss is clinically characterized by widening of midline parting or diffuse central or vertex/frontal (male pattern) along with sparing of the occiput (8). In a study conducted by Ramos et al. of dermoscopic patterns in AGA among females, out of 34 patients enrolled in their study, all had vellus hair, 64.7% had peri follicular pigmentation, and 41.18% had honeycomb pigmentation (9). Rakowska et al. criteria are used for diagnosing FPHL; however, we could not use them (10). Saqib et al. found that increased hair diameter diversity > 20%, single-hair follicular unit, vellus hair, peripilar sign, and focal atrichia were commonly seen in female pattern hair loss on trichoscopy (11).
Alopecia areata (AA) is an immune-mediated condition. Waskiel et al., in the review article on trichoscopic features of alopecia areata, reported that the common features were yellow dots (6 - 100% patients), short vellus hairs (34 - 100%), black dots (0 - 84%), broken hairs (0 - 71%) and exclamation mark hairs (12 - 71%). Rarely reported features, which include upright regrowing hairs (11 - 96%), pigtail (circle) hairs (4 - 61%), and Pohl-Pinkus constrictions (2 - 10%), may also be helpful in the diagnosis of alopecia areata. There is no pathognomonic trichoscopic marker for alopecia areata, and the most common trichoscopic features are not the most specific (4). However, in our study, most subjects had vellus hair and yellow dots, and black dots.
Rajan et al. concluded that the sensitive markers for alopecia areata are short vellus hair and yellow dots, and the specific markers are exclamatory hair, broken hairs, black dots, and tapering hair (12). The disappearance of black dots, broken hairs, and exclamation mark hairs indicates a good response to treatment. By contrast, the observation of yellow dots indicates chronic disease and poor response to treatment (13). All our patients were treatment naïve.
Bhat et al. conducted a study evaluating the dermoscopic features in dermatophyte infection, where ten subjects had comma hairs alone or with corkscrew hairs among other dermoscopic features like zigzag hairs, black dots, short vellus, barcode (morse code hairs) (14). These findings are similar to the findings seen in our study. Lim et al. reported that trichoscopy aids in detecting tinea capitis with a sensitivity of 94% and specificity of 83% (15). A recent systematic review on trichoscopic patterns of tinea capitis reported that trichoscopy could be potentially utilized in differentiating the causative agent of tinea capitis from Microsporum species or Trichophyton species, thus, increasing the accuracy of diagnosis and early initiation of treatment (16).
A systematic review by Zychowska and Zychowska on dermoscopic patterns in discoid lupus erythematosus (DLE) showed that in scalp DLE (n = 166), the most common findings were: White structureless areas (62%), arborizing vessels (57.8%), white scales (54.2%), follicular keratotic plugs (47%), absent follicular openings (45.8%), perifollicular scaling (43.9%), speckled brown pigmentation (38%) which were comparable to the findings seen in our study (17).
A study by Ankad et al. on trichoscopy in trichotillomania showed that out of ten patients included in the study, the common trichoscopic feature was decreased hair density and broken hairs along with features like trichoptilosis and irregularly coiled hairs, which were seen in 80% patients (18). While in our study, splaying of hair with other features like scaling, yellow dots, and black dots were seen.
5.1. Limitations
Small sample sizes and skin biopsies could not be done on all patients.
5.2. Conclusions
This study showed that dermoscopic diagnosis was accurate in ambiguous cases of alopecia. Thus, trichoscopy is a non-invasive tool that has a definite role in early diagnosis and avoiding unnecessary invasive procedures and early initiation of treatment. It also helps to monitor the response to treatment with more accuracy.