1. Background
Critically-ill patients receiving care in intensive care units (ICU) often present complicated cases whose immobility, pharmacological treatments, sweating, and reduced perfusion compromise skin integrity (1, 2). Skin, being the largest body organ, forms the primary interface between internal organs and the external environment, providing a physical and immunological barrier (3).
Protective molecules in the epidermis and inflammatory mediators produced by keratinocytes regulate skin immune responses (4). Cutaneous antimicrobial defenses rely on surface lipids, skin surface acidification, iron-binding proteins, and antimicrobial peptides (5). The permeable barrier function of the skin impedes the transcutaneous movement of water and electrolytes (3), and impairment in this function increases susceptibility to adverse events like infections and allergies. Irvine introduced the concept of skin failure, considering it equivalent to other vital organ dysfunctions (6).
It is pertinent to study dermatological disorders (DDs) in ICU patients as their detection may influence management strategies, the patient’s quality of life, duration of ventilation, length of hospitalization, patient outcomes, and the mortality rate (1, 7).
Agrawal et al. found that dermatological manifestations in patients were associated with higher illness severity and increased mortality. Also, mortality rates were higher in patients with primary dermatological conditions requiring ICU admission (65.6%) and those with manifestations resulting from critical illnesses (57.5%) compared to those without any DDs (35.5%). Dermatological manifestations also correlated with longer ventilation duration and extended ICU and hospital stays (7).
Severe DDs, like adverse drug-induced cutaneous reactions, may themselves lead to ICU admission (2). The timely detection and correct classification of DDs are key to designing appropriate care and treatment plans in ICUs (8).
Various parameters, such as age, gender, duration of ICU stay, comorbidities, treatment strategies, and underlying systemic disorders, may predispose patients to cutaneous manifestations (1, 9, 10). Hence, it is essential to scrutinize the predictors of various DDs in ICU-admitted patients.
Currently, there is a paucity of published Indian literature (7, 10) on this important aspect of ICU patient management, and minimal data are available from our hospital, which houses 96 ICU beds and faces a high turn-over rate and an average bed-occupancy rate of 100%.
2. Objective
The primary objective/outcome was to determine the prevalence of DDs among critically ill patients. Additionally, the study sought to document and classify these disorders and to assess the correlation of various parameters (age, gender, length of hospital stay, comorbidities, and time to consultation) with the type of DD.
3. Methods
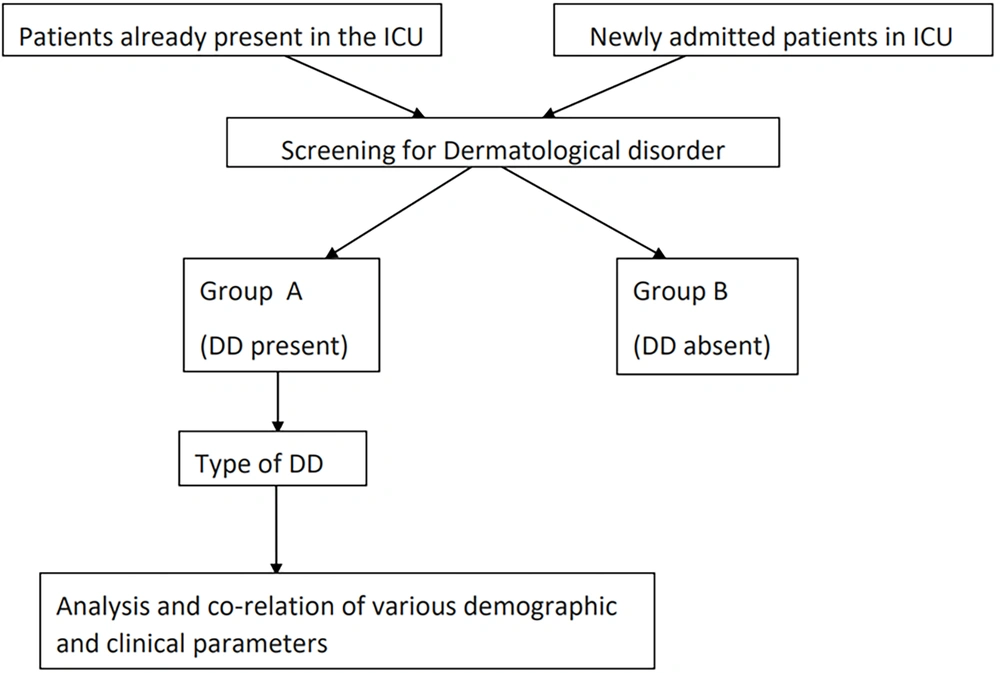
This hospital-based observational cross-sectional study included all patients admitted to the medicine (MICU), paediatric (PICU), and neonatal (NICU) intensive care units of a tertiary care teaching hospital in Pune, India, over two months (Figure 1). Written informed consent was obtained from the legal guardian/primary caregiver (or attending doctor in the absence of a legal guardian).
Patients whose details were incomplete and those with pre-existing skin lesions that were not a cause of ICU admission were excluded.
Permission for conducting the project was obtained from the institutional ethics committee.
All patients (or relatives) were assured of confidentiality.
3.1. Definitions
3.1.1. Length of ICU Stay
Time duration from the day of ICU admission until the appearance of the skin lesion (for patients with primary skin lesions leading to ICU admission (i.e., Type 3), the length of ICU stay was set to zero as the patient directly came with a developed skin lesion).
3.1.2. Comorbidity
The presence of any underlying major illness apart from the primary diagnosis leading to ICU admission (for example, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), etc.).
Screening for DDs was conducted within 48 hours of admission, and hospital records were reviewed for patients already hospitalized in the ICU at study initiation. The diagnosis of skin lesions was made by one of the investigators (the second author), who is an associate professor and the head of the dermatology unit with a post-graduate qualification (MD dermatology). The daily screening was conducted throughout the ICU stay until discharge or death.
Prevalence = (Number of patients with DDs × 100)/Total patients screened.
Burden of skin lesion = (Number of skin lesions × 100)/Total patients screened.
Record sheets of patients were reviewed, and requisite information was recorded using a predesigned proforma. The parameters gathered included: Age, gender, type of ICU, registration number, dermatological condition, length of ICU stay, primary diagnosis leading to ICU admission (cardiovascular disease/central nervous system disease/respiratory system disease/abdominal and pelvis system disease/hematological disease/multi-systemic disease/primary DD), comorbidities (diabetes mellitus/hypertension/coronary artery disease/COPD/malignancy/chronic renal failure/stroke, others), the presence of single or multiple comorbidities, and birth weight (low birth weight (LBW)/very LBW/extremely LBW) for neonates.
Interviews with patients’ relatives were conducted to determine the onset, duration, and stage of DD at ICU admission.
Dermatological disorders were categorized as type 1 (dermatological infections), type 2 (iatrogenic DDs), type 3 (primary DD leading to ICU admission), type 4 (primary ICU-acquired DDs unrelated to the primary disease), type 5 (primary DD occurring in the ICU secondary to the primary disease), type 6 (miscellaneous), type 7 (infectious lesions secondary to the primary disease).
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) software version 20:0. Qualitative variables were expressed as frequency and percentage. Quantitative variables were expressed using mean and standard deviation.
The chi-square test and Fisher’s exact test were used to scrutinize the association between categorical variables. P values < 0.05 were considered statistically significant.
4. Results
A total of 688 patients were screened in the MICU (304), PICU (100), and NICU (284). Of these, 129 patients in all ICUs had 136 skin lesions, comprising 74 lesions among 69 patients in the MICU, 12 lesions in 12 patients in the PICU, and 50 skin lesions in 49 patients in the NICU, giving a combined prevalence of 18.9% and an overall burden of skin lesions of 19.8%. The MICU had the highest prevalence with 22.7% (a burden of 23.7%), and PICU and NICU had a prevalence of 12% and 17.3%, respectively (a burden of 17.6%). Dermatology consultation was requested for 42 patients (32.6%), constituting 19 (25.7%), 9 (75%), and 14 (28%) patients in MICU, PICU, and NICU, respectively.
A significant association was observed between the type of the skin lesion and age in the MICU, where patients under 30 years old predominantly presented with type 3 (i.e., primary DDs leading to ICU admission) and type 5 (DDs developing due to the underlying disease in the ICU) lesions. Patients in the age group of 31-50 years mostly showed type 7 lesions (i.e., infectious lesions secondary to the primary disease), while 51-70-year-old patients presented with type 1 conditions (i.e., infectious lesions), and patients over 70 years old were mostly identified with type 2 lesions (i.e., iatrogenic lesions).
Also, the presence or absence of comorbidities was significantly associated with the type of skin lesion. The absence of any comorbidity was associated with type 1 (i.e., infectious) and type 2 (i.e., iatrogenic) lesions. The presence of isolated comorbidity was associated with type 2 (iatrogenic) and type 5 (DDs developing due to the underlying disease in the ICU) lesions, while the presence of multiple comorbidities was associated with type 2 (iatrogenic) conditions. Direct admission to the ICU was significantly associated with type 4 lesions (i.e., primary DDs occurring in the ICU), while prior admission to the ward was associated with type 5 lesions (i.e., DDs caused by the underlying disease in the ICU).
Table 1 depicts the distribution and classification of various DDs. Table 2 shows the relationship between age groups and the skin lesion type in the MICU (P = 0.004). In this regard, type 3 and type 5 lesions were significantly more prevalent in patients under the age of 30 years old, type 7 lesions in the age group of 31 - 50 years, type 1 lesions among 51 – 70 year-olds, and type 2 lesions in patients over 70 years of age.
| Category of DD | Type | MICU | PICU | NICU |
|---|---|---|---|---|
| 1 | Dermatological infections | 11 | 9 | 17 |
| 1A | Bacterial infections | 4 | 1 | 14 |
| 1B | Fungal infections | 5 | 1 | 3 |
| 1C | Viral infections | 2 | 7 | 0 |
| 2 | Iatrogenic DD | 40 | 1 | 13 |
| 2A | Due to drugs administered in ICU | 9 | 0 | 0 |
| 2B | Due to procedures performed in ICU | 24 | 1 | 13 |
| 2C | Others (Due to the bedridden status of patients) | 7 | 0 | 0 |
| 3 | Primary DD as a cause of ICU admission | 2 | 1 | 3 |
| 4 | Primary DD occurring in ICU (unrelated to the disease) | 0 | 0 | 11 |
| 5 | Primary DD occurring in ICU secondary to the disease | 16 | 1 | 1 |
| 6 | Miscellaneous | 0 | 0 | 5 |
| 7 | Infectious lesions secondary to primary disease | 5 | 0 | 0 |
Distribution of Patients in the ICUs Based on the Type of Dermatological Disorder
| Type of Lesion | NICU | PICU | MICU | |||||
|---|---|---|---|---|---|---|---|---|
| < 8 Days | > 7 Days | < 25 Months | > 24 Months | < 30 Years | 31 - 50 Years | 51- 70 Years | > 70 Years | |
| Type 1 | 4 (23.5) | 13 (76.5) | 4 (44.4) | 5 (54.6) | 2 (18.2) | 2 (18.2) | 7 (63.6) | 0 |
| Type 2 | 5 (38.5) | 8 (61.5) | 0 | 1 (100) | 7 (17.5) | 4 (10) | 20 (50) | 9 (22.5) |
| Type 3 | 12 (85.7) | 2 (14.3) | 1 (100) | 0 | 1 (50) | 0 | 1 (50) | 0 |
| Type 4 | 7 (63.6) | 4 (36.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Type 5 | 0 | 1 (100) | 0 | 1 (100) | 8 (50) | 4 (25) | 4 (25) | 0 |
| Type 6 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Type 7 | 0 | 0 | 0 | 0 | 0 | 4 (80) | 1 (20) | 0 |
Age-wise Distribution of Patients with Respect to the Type of the Skin Lesion a
According to Table 3, there was a significant association between the skin lesion type and the presence/absence of comorbidities (P = 0.004). The absence of any comorbidity was linked to type 1 and type 2 lesions, while the presence of a single comorbidity was associated with type 2 and type 5 lesions. Multiple comorbidities coincided with type 2 lesions.
As shown in Table 4, a significant association was observed between direct admission to ICU and type 4 lesions, while prior admission to ICU was linked with type 5 lesions. Finally, the type of skin lesion was significantly associated with the length of stay in the PICU (Table 5, P < 0.001), and patients with type 2 lesions had the longest stay.
| Type of Lesion | No Comorbidity | Single Comorbidity | Multiple Comorbidity | Total |
|---|---|---|---|---|
| Type 1 | 32 (33.7) | 4 (13.8) | 1 (8.3) | 37 |
| Type 2 | 34 (35.8) | 13 (44.8) | 7 (58.3) | 54 |
| Type 3 | 4 (4.2) | 0 | 2 (16.7) | 6 |
| Type 4 | 10 (10.5) | 1 (3.4) | 0 | 11 |
| Type 5 | 10 (10.5) | 8 (27.6) | 0 | 18 |
| Type 6 | 4 (4.2) | 1 (3.4) | 0 | 5 |
| Type 7 | 1 (1.1) | 2 (6.9) | 2 (16.7) | 5 |
| Total | 95 | 29 | 12 | 136 |
Distribution of Dermatological Disorders Based on the Presence or Absence of Comorbidities a
| Type of Lesion | Direct Admission to the ICU | Prior Stay in a Ward Before ICU Admission |
|---|---|---|
| Type 1 | 23 (25.6) | 14 (30.4) |
| Type 2 | 36 (40) | 18 (39.1) |
| Type 3 | 5 (5.6) | 1 (2.2) |
| Type 4 | 11 (12.2) | 0 |
| Type 5 | 8 (8.9) | 10 (21.7) |
| Type 6 | 5 (5.6) | 0 |
| Type 7 | 2 (2.2) | 3 (6.5) |
| Total | 90 | 46 |
Distribution of Dermatological Disorders Based on the Route of ICU Admission a
| Type of Lesion | NICU | PICU | MICU | |||
|---|---|---|---|---|---|---|
| No. of Patients | MLS | No. of Patients | MLS | No. of Patients | MLS | |
| Type 1 | 17 | 11.88 | 9 | 3.89 | 11 | 6.91 |
| Type 2 | 13 | 5.69 | 1 | 18.00 | 40 | 9.08 |
| Type 3 | 3 | 0.00 | 1 | 0.00 | 2 | 0.00 |
| Type 5 | 11 | 3.82 | 1 | 3.00 | 16 | 7.13 |
| Type 7 | 1 | 4.00 | 12 | 4.67 | 5 | 5.00 |
Distribution of Dermatological Disorders in ICUs Based on the Mean Length of Stay
5. Discussion
We analyzed dermatoses in critically ill patients admitted to ICUs at a tertiary teaching hospital and found a higher prevalence of DD in the MICU compared to PICU/NICU, which could probably be artefactual owing to a larger number of patients screened in MICU or due to differences in age-related factors or underlying comorbidities. A literature search did not reveal any previous study assessing this type of data across three different ICU settings in a single hospital. Overall, the prevalence of DDs in our study was higher than those previously documented. Badia et al. (8) reported a prevalence of 9.2% in a population, half of whom were surgical/trauma patients. In two separate but similar studies among medical ICU patients, Emre et al. (9) and Lee et al. (3) reported that dermatology consultation was requested for 13.9% and 1.2% of the participants, respectively. Immobility and multiple co-existing diseases like diabetes (which is known to predispose to skin lesions) may explain the higher prevalence of cutaneous manifestations in our study.
Although we found no relationship between age and the prevalence of DD, a statistically significant association was noticed between age and the type of DD. Elderly individuals who were in their sixth and seventh decades of life were more likely to acquire infectious DDs (bacterial furuncle/impetigo/cellulitis, superficial fungal infections, and viral infections such as herpes labialis and herpes zoster). On the other hand, younger individuals in their third decade of life were more likely to develop skin lesions secondary to an underlying disease (e.g., cellulitis secondary to diabetes or hepatorenal syndrome, metabolic syndrome, or oral candidiasis secondary to tuberculosis). This observation was consistent with the findings of Pektas and Demir (1) in a study on surgical ICU patients. With aging, the flattening of dermal papillae at the dermo-epidermal junction and reduced density of dermal elastin and collagen fibers can shrink skin thickness. Wollina and Novak (2) reported that aging facilitates the occurrence of DDs, as well as the emergence of drug-induced cutaneous reactions. Thus, impaired skin barrier and immune function in older individuals predisposes them to cutaneous infections.
Males constituted a larger proportion of our patients in this study; however, there was no statistically significant correlation between gender and the type of DD. Emre et al. (9) found that cutaneous drug reactions were more frequent in female patients. The diminishing protective effect of estrogen after menopause may explain the greater propensity of peri-menopausal females to dermatoses.
Overall, iatrogenic dermatoses were foremost in the MICU, followed closely by infectious lesions. Iatrogenic lesions included skin eruptions resulting from the drugs administered (e.g., aspirin-induced ecchymoses, furosemide-induced lichenoid drug eruption) and the procedures performed (thrombophlebitis at the venepuncture site) during ICU admission. Such dermatoses have not been reported in previous studies on critically ill adult patients. This finding of ours emphasizes the need for aseptic precautions during procedures like venepuncture, arterial puncture, cannulation, and central venous line insertion in ICUs. Regular appraisal and improvement of ICU personnel’s skills are essential. Although the resulting lesions may seem minuscule, ignoring them can lead to serious complications, including thrombosis and cellulitis.
Infections such as varicella and herpes simplex in infants and children and superficial pyodermas in neonates were predominant dermatoses in PICU and NICU. The urgent upscaling of infection control measures is needed to prevent infections in these vulnerable populations who generally have compromised immune systems and comorbidities.
According to previous studies, an increase in the prevalence of DDs has been associated with diabetes, chronic renal failure, cardiovascular disorders, and immunosuppressive drug use. Fischer et al. (11) and Badia et al. (8) suggested a link between the use of immunosuppressives and corticosteroids and infectious dermatoses, which also explains the high prevalence of cutaneous infections in our subjects. The frequency of DDs was relatively higher in patients with multiple comorbidities, indicating that the presence of multiple comorbidities could deliver patients predisposed to DDs depending on the type of underlying medical conditions. On the other hand, those with only a single primary disease had fewer DDs. This was not surprising as many medical conditions like diabetes, septicemia, hepatobiliary problems, renal disease, and hematological derangements are known to present with cutaneous manifestations like icterus, petechiae, and purpura fulminans. Therefore, a thorough skin examination can provide valuable insights into the patient’s health and prognosis.
The median length of stay in our study was five days for the MICU and NICU and four days for the PICU. A longer stay was associated with the development of iatrogenic DDs. Pektas and Demir (1) reported a median length of stay of seven days in the surgical ICU with a higher prevalence of DDs in patients admitted beyond ten days; however, they could not demonstrate a link between the DD subtype and the duration of stay. Prior studies have linked a longer ICU stay with the increased incidence of DDs, particularly in patients who finally succumbed to the condition. Our study’s cross-sectional design precluded assessing the impact of ICU stay duration on patient outcomes (death or discharge). Longer ICU stays generally encompass complex therapies, invasive procedures, and a higher risk of infections. Increased exposure to drugs and disinfectants heightens the risk of drug reactions, and the co-existence of other conditions can prolong ICU stay and increase mortality rates. Although we found no link between the skin lesions observed in the PICU and NICU and the length of stay, the onset of these lesions was similar in terms of the time of occurrence in all three ICUs.
We also analyzed the relationship between the route of admission to the ICU and DD type and discovered that patients admitted directly to the ICU were more likely to develop non-ICU-related DDs (Erythema Toxicum Neonatorum (ETN)), while those transferred from other wards were more prone to DDs related to their primary illnesses (e.g., trophic ulcer overlying meningomyelocele in a child with hydrocephalus). Longer stays in other wards before ICU admission could exacerbate infectious DDs, an aspect of critical care that is under-researched, warranting further investigations.
Primary skin conditions requiring intensive care are relatively rare. We encountered six patients with DDs necessitating ICU admission, including two patients with Stevens-Johnson syndrome (SJS) in MICU, one child with extensive cutaneous lesions due to Langerhans cell histiocytosis (later detected with multi-organ involvement) in PICU, and three neonates (epidermolysis bullosa, progeria, and extensive impetigo) in NICU.
In our study, the prevalence of neonatal dermatoses was 17.6%, which was much lower than that reported in other studies. Shehab reported a prevalence of 74.6% (12). We found a higher proportion of acquired and iatrogenic disorders compared to physiological and developmental disorders. The predominant type of acquired disorders included bacterial infections, followed by fungal (candidal) conditions, while the foremost physiological condition encountered was ETN (22%). Iatrogenic complications like ecchymoses and thrombophlebitis were noted on the dorsum of hands and ankles at the sites of intravenous cannula insertion or venepuncture. Phototherapy-induced erythema was noticed in two neonates with hyperbilirubinemia, constituting a greater proportion of neonatal dermatoses in our study in comparison with other studies (13, 14). Contrary to this, Naveen et al. (15) found a higher frequency of physiological dermatoses and demonstrated that iatrogenic lesions were more frequent in male pre-term, LBW neonates and the late neonatal period. Diaper dermatitis, which is a common condition in neonates, was found only in one child. This was much lower compared to previous studies from India (4%, Naveen et al. (15)), Egypt (15.2%, Shehab et al. (12)), and Pakistan (15.59%, Javed (16)). The lower prevalence in our study is an encouraging finding attributable to the optimum frequency of diaper change and judicious use of topical agents with potential for causing irritant and allergic dermatitis.
The strength of our study lies in the thorough screening of a large number of patients across three different ICUs over a short duration, as well as documenting similarities and differences between diverse age groups. We assessed a wide range of demographic and disease-related variables, which is a notable advantage respective to previous studies that evaluated only individual ICUs (either MICU (6, 8, 9, 17)), PICU (1, 13, 15, 18), or NICU (12, 14, 16, 19, 20)).
Limitations of this study include the lack of a control group for comparisons between patients with and without DDs. This pilot study provided an overview of the burden of DDs in critically ill patients. Although some variables showed a correlation with the frequency or type of DDs, their impact on prognosis remained unclear, warranting further prospective studies on larger sample sizes and in-depth analyses.
5.1. Conclusions
This study brought to light that DDs were frequent in ICU-admitted patients and were related to factors such as age, length of stay, and route of admission. Our findings provided a practical classification and described common skin lesions in MICU, OICU, and NICU. While the spectrum of dermatoses was similar across these ICUs, the proportional distribution differed by age group. Early diagnosis and treatment are imperative for improving patient outcomes, necessitating boosting the awareness of ICU personnel and dermatologists along with regular skin examinations.