1. Background
Vitiligo is a prevalent autoimmune pigmentary condition of the skin, with an estimated prevalence of 0.5 - 2% worldwide (1). The disease specifically attacks melanocytes, leading to the appearance of milky white macules and patches (2). The exact etiology is unknown, which is a major issue that must be addressed to facilitate the creation of novel, individualized therapeutic strategies (3). Human follicular T helper cells, dendritic follicular cells, and mesenchymal lymphoid tissue organizer cells all generate CXCL13, chemokine (C-X-C motif) ligand 13, known as B lymphocyte chemoattractant (BLC) or B cell-attracting chemokine 1 (BCA-1). The activation of CXCL13 and its receptor CXCR5 have been linked to the pathogenesis of various autoimmune and inflammatory diseases, including systemic lupus erythematosus, rheumatoid arthritis, and autoimmune thyroiditis (4).
Chemokine (C-X-C motif) ligand 13 is vital in lymphoid neogenesis and immune responses. It is also involved in the initiation and organization of ectopic lymphoid-like structures (ELSs), the organized lymphocyte aggregates developed at sites of inflammation in target tissues of autoimmune diseases. In ELSs of inflammatory tissues, the elevated expression of CXCL13 can regulate B-cell infiltration and shuttling inside the ectopic germinal centers (GCs). The pivotal role of the CXCL13/CXCR5 axis in ELS development has been detected in several autoimmune diseases, indicating that CXCL13 might attract B cells and contribute to the formation of ELSs in chronic inflammation (5).
Chemokine (C-X-C motif) ligand 13 has been found to be higher in psoriasis vulgaris patients and positively correlated with the disease severity; accordingly, the axis CXCL13/CXCR5 might contribute to the pathogenesis of psoriasis and constitute new targets in the treatment of psoriasis (6).
2. Objectives
To the best of our knowledge, the CXCL13 serum level has not been assessed before in vitiligo patients. Therefore, this study aimed to evaluate the CXCL13 serum level in patients with vitiligo and to correlate it with the activity and severity of vitiligo. Consequently, this study can provide insight into vitiligo etiopathogenesis and constitute new targeted strategies for treatment.
3. Methods
This case-control study compared 21 vitiligo patients and 21 age and gender-matched controls. Patients were recruited from the Dermatology Department Outpatient Clinic, Faculty of Medicine, Suez Canal University Hospital, Ismailia, Egypt. The study was conducted by the Clinical Pathology Department of the same institution. The study was carried out within March and October 2021, following the guidelines laid out in the STROBE declaration and the principles outlined in the Helsinki Declaration. According to ethical clearance, the institutional review board (IRB) and the Research Ethics Committee of Suez Canal University gave their clearance on January 18, 2021, number 4445. Participants who had a history of autoimmune conditions, atopy, malignancy, pregnancy, viral infections, or under immunosuppressive medicines were not included in the study. Everyone involved signed a written statement of informed consent.
Vitiligo was diagnosed based on the clinical evaluation of symmetrically distributed depigmented macules or patches. On Wood’s lamp inspection, a milky white look supported the diagnosis. Due to the lesions’ usual clinical appearance, none of the patients had a biopsy. Both male and female patients with non-segmental vitiligo were included. Patients who had local treatment in the previous 2 weeks and phototherapy or systemic medication in the previous 3 months were excluded.
Patient’ histories were obtained, which contained essential demographic information (i.e., age, gender, address, and occupation) and data from the medical field (i.e., age of onset, course of illness, duration, location, incidence of progression of existing lesions, appearance of new lesions, previous treatment received and response to it, and a history of vitiligo or other autoimmune diseases in the family). Vitiligo’s location, severity, and pattern were evaluated by dermatological examination. The Vitiligo Extent Score (VES) was used to gauge the severity of the vitiligo (7). The Vitiligo Disease Activity (VIDA) (8) score was used to assess the activity of vitiligo, and it was evaluated as follows:
Activity in the last 6 weeks or less (+4), the last 6 weeks to 3 months (+3), and the past 6 months (+2)
A score of +2 indicates activity in the last 3 - 6 months, +1 indicates activity in the past 6 - 12 months, 0 indicates stability for 1 year or longer, and 1 indicates 1 year or longer for stability with spontaneous regimentation. Additionally, the disease activity clinical signs, such as the Koebner phenomenon, depigmentation that resembled confetti, and ill-defined borderlines, were assessed in patients (9).
The serum levels of CXCL13 were determined by enzyme-linked immunosorbent assay (ELISA) kit Cat. No E0062Hu (Bioassay Technology Laboratory, Shanghai Korain Biotech Co Ltd, China), following the manufacturer’s instructions. A standard curve was created ranging from 10 - 2000 ng/L with a sensitivity of 4.62 ng/L by the professional software “CurveExpert 1.4”, and the mean values were calculated.
3.1. Statistical Analysis
The data of this study were analyzed by SPSS software (version 20.0; IBM Corp., Armonk, New York, the USA). The data were expressed as mean and standard deviation or number and frequencies according to the type of variables. The chi-square and t-test were used to compare qualitative and quantitative variables, respectively. In cases where the descriptive data were highly skewed, the Mann-Whitney U test and the Kruskal-Wallis test were used. The Spearman and Pearson’s correlation coefficients were used to analyze the degree of association. When the P-value was less than 0.05, the difference was statistically significant.
4. Results
A total of 21 Egyptian vitiligo patients (9 men and 12 women; mean age: 35.33 ± 17.76 years; range: 7 - 68 years) and 21 Egyptian healthy participants (7 men and 14 women; mean age: 28.19 ± 13.37 years; range: 9 - 61 years) participated in the study. There was no statistically significant difference between both groups regarding age and gender. Table 1 shows the clinical features of vitiligo patients. Vitiligo vulgaris was the most common pattern, appearing in 14 patients (66.7%). Other patterns were present in lesser numbers. The mean VES was 13.99 ± 27.35 (range: 0.06 - 97.88). The most common VIDA score was VIDA +4 (n = 13, 61.9%). Additionally, 8 patients showed all the clinical symptoms of activity, including the Koebner phenomenon, confetti-like depigmentation, and poorly defined boundary (38.1%).
| Variables | Vitiligo Patients (n = 21) |
|---|---|
| Age of onset (y) | |
| Mean ± SD | 13.50 ± 12.75 |
| Median (IQR) | 13.0 (3.0 - 22.0) |
| Duration of the disease (y) | |
| Mean ± SD | 13.50 ± 12.75 |
| Median (IQR) | 13.0 (3.0 - 22.0) |
| Clinical subtypes, No. (%) | |
| Vulgaris | 14 (66.7) |
| Facial | 2 (9.5) |
| Universalis | 1 (4.8) |
| Acrofacial | 2 (9.5) |
| Vulgaris, acrofacial | 2 (9.5) |
| Clinical signs of activity, No. (%) | |
| No | 3 (14.3) |
| Poorly defined border | 3 (14.3) |
| Koebner phenomenon, confetti-like depigmentation | 2 (9.5) |
| Confetti-like depigmentation, poorly defined border | 5 (23.8) |
| Koebner phenomenon, confetti-like depigmentation, poorly defined border | 8 (38.1) |
| Family history of vitiligo, No. (%) | |
| Negative | 13 (61.9) |
| Positive | 8 (38.1) |
| VES score | |
| Mean ± SD | 13.99 ± 27.35 |
| Median (IQR) | 3.11 (0.42 - 9.29) |
| VIDA score, No. (%) | |
| -1 | 1 (4.8) |
| 0 | 2 (9.5) |
| +1 | 0 (0.0) |
| +2 | 3 (14.3) |
| +3 | 2 (9.5) |
| +4 | 13 (61.9) |
Abbreviations: SD, standard deviation; IQR, interquartile range; VES, Vitiligo Extent Score; VIDA, Vitiligo Disease Activity.
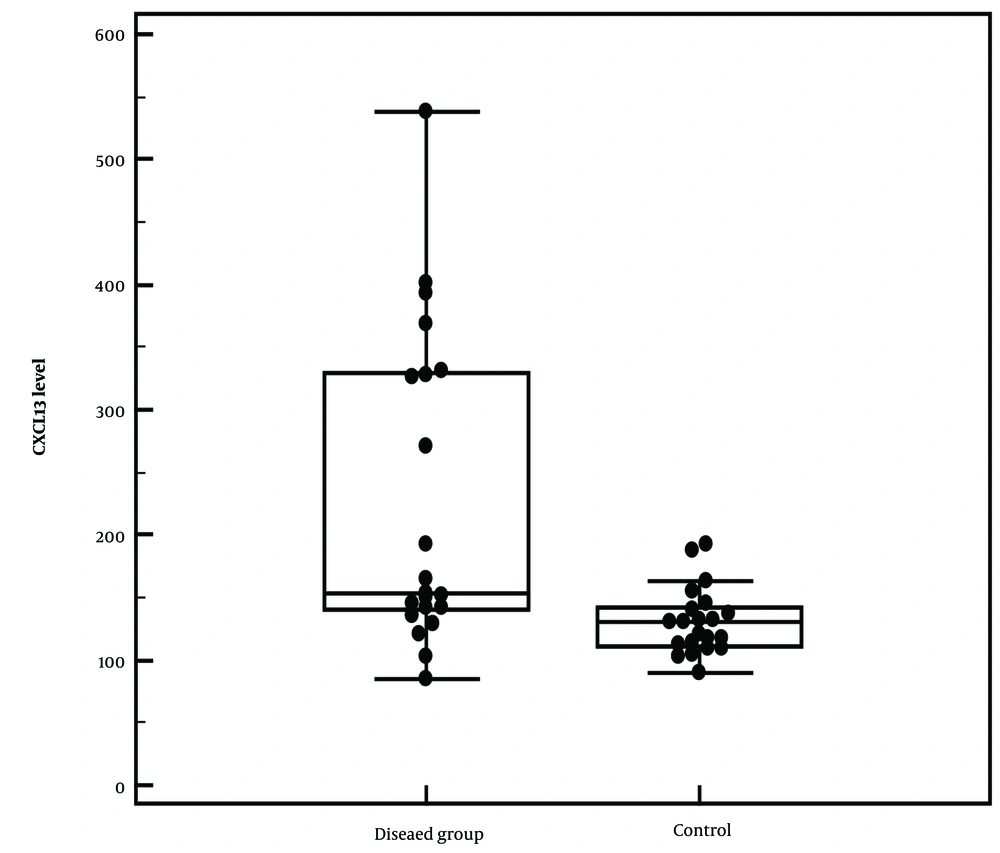
In comparison to healthy controls, patients with vitiligo showed significantly higher blood levels of CXCL13 (mean: 227.35 ± 125.70 ng/L, range: 85.59 - 538.38 ng/L) (P = 0.002*) (Figure 1). Moreover, there was a statistically significant (P = 0.043*) relation between the CXCL13 level and the clinical pattern of activity among vitiligo patients, with a mean of 433.28 ± 148.63 ng/L (± SD); individuals who displayed both vulgaris and acrofacial patterns had the greatest level. However, considering the activity, the clinical signs of the activity, and the VIDA score, there was no significant correlation (Table 2). Moreover, there was no significant difference between CXCL13 and demographic data among vitiligo patients corresponding to the age, gender, age of onset, duration, or family history of the disease. No correlation was observed between CXCL13 with age, age of onset, VIDA, and VES scores (Table 3).
| Variables | No | CXCL13 Level (ng/L) | Test of sig. a | P-Value b | ||
|---|---|---|---|---|---|---|
| Min-Max | Mean ± SD | Median | ||||
| Clinical pattern | H = 8.147 c | 0.043 c | ||||
| Vulgaris | 14 | 85.59 – 368.91 | 191.64 ± 92.48 | 150.63 | ||
| Facial | 2 | 135.35 – 165.47 | 150.41 ± 21.29 | 150.41 | ||
| Universalis | 1 d | 128.73 | ||||
| Acrofacial | 2 | 393.68 – 401.63 | 397.65 ± 5.62 | 397.65 | ||
| Vulgaris, acrofacial | 2 | 328.19 – 538.38 | 433.28 ± 148.63 | 433.28 | ||
| Clinical signs of activity | H =4.215 | 0.378 | ||||
| No | 3 | 128.73 – 192.58 | 162.26 ± 32.04 | 165.47 | ||
| Poorly defined border | 3 | 85.59 – 331.09 | 173.38 ± 136.88 | 103.45 | ||
| Koebner phenomenon, confetti-like depigmentation | 2 | 121.26 – 151.52 | 136.39 ± 21.39 | 136.39 | ||
| Confetti-like depigmentation, poorly defined border | 5 | 135.35 – 368.91 | 248.90 ± 106.55 | 269.90 | ||
| Koebner phenomenon, confetti-like depigmentation, poorly defined border | 8 | 141.59 – 538.38 | 281.27 ± 154.48 | 240.32 | ||
| Activity | U = 23.0 | 0.740 | ||||
| No | 3 | 128.73 – 192.58 | 162.26 ± 32.04 | 165.47 | ||
| Yes | 18 | 85.59 – 538.38 | 238.20 ± 132.65 | 152.71 | ||
| VIDA | H = 1.192 | 0.755 | ||||
| (-1) Stable for 1 year or more with spontaneous regimentation | 1 d | 192.58 | ||||
| (0) Stable for 1 year or more | 2 | 128.73 – 165.47 | 147.10 ± 25.97 | 147.10 | ||
| (+1) Activity in the last 6-12 months | 0 | – | – | – | ||
| (+2) Activity in the last 3-6 months | 3 | 85.59 – 328.19 | 186.08 ± 126.54 | 144.48 | ||
| (+3) Activity in the last 6 weeks to 3 months | 2 | 142.17 – 331.09 | 236.63 ± 133.59 | 236.63 | ||
| (+4) Activity in the last 6 weeks or less | 13 | 103.45 – 538.38 | 250.47 ± 141.17 | 153.90 | ||
Abbreviations: CXCL13, chemokine (C-X-C motif) ligand 13; VIDA, Vitiligo Disease Activity Index; SD, standard deviation.
a H for the Kruskal-Wallis test; U for the Mann-Whitney U test.
b P-value for comparing different categories.
c Statistically significant at P ≤ 0.05
d Excluded from the comparison due to the small number of cases (n = 1)
| Variables | CXCL13 Level (ng/L) | |
|---|---|---|
| rs a | P-Value | |
| Age (y) | -0.016 | 0.946 |
| Age of onset (y) | 0.116 | 0.617 |
| VIDA | -0.006 | 0.978 |
| VES score | 0.167 | 0.470 |
Abbreviations: CXCL13, chemokine (C-X-C motif) ligand 13; VES, Vitiligo Extent Score; VIDA, Vitiligo Disease Activity.
a rs: Spearman’s coefficient
5. Discussion
Vitiligo is an autoimmune pigmentary disorder that, due to the absence of functioning melanocytes, manifests as white macules and patches affecting the skin, mucous membrane, and/or hair. Regardless of age, color, or ethnicity, it affects both genders and accounts for about 1% of the global population (10). The current study indicates that several processes, including oxidative stress, metabolic dysfunction, and immunological response, are involved in the complicated etiopathogenesis of vitiligo. The start of vitiligo is associated with several triggers, including mechanical stimuli and chemical exposures. These triggers are expected to result in stress responses in keratinocytes and melanocytes, which are assumed to be the source of the imbalance of the oxidative and antioxidant systems (2). However, there is still disagreement about the precise causes of this chain of events because mounting evidence points to the CXCL13/CXCR5 signaling axis as a possible initiator of several autoimmune diseases, including SLE and RA. Nevertheless, research on CXCL-13’s function in vitiligo is still lacking (5).
This study detected a significant difference between the serum levels of CXCL13 in patients (227.35 ± 125.70 ng/L) compared to the controls (130.58 ± 26.52 ng/L). Liu et al. (6) studied the function of CXCL13 in the etiology of psoriasis and discovered that patients’ levels were substantially higher than controls.
As for SLE, Fang et al. (11) observed that serum CXCL13 levels were significantly elevated among SLE patients, compared to healthy controls. They also stated that not only was there a significantly positive association between CXCL13 levels and the SLE Disease Activity Index but also an inverse correlation between CXCL13 concentration and memory B-cell count. Accordingly, they concluded that CXCL13 might be used as a practical tool in the judgment of active SLE.
This study postulates that the role of CXCL13 in vitiligo etiopathogenesis could be similar to CXCL12. The reason for this assumption is that both chemokines belong to the same functional family: homeostatic chemokines that are responsible for basal leukocyte migration (12). He et al. (13) noted that vitiligo patients had serum levels of CXCL12 considerably higher than healthy controls. They also showed that CXCL12 has been identified as a key mediator for activating Langerhans cells and interferon (IFN)-producing cells in the skin. The in vivo animal investigations further proved that CXCL12 boosted the recruitment of T cells and antigen-presenting cells (APCs) to the area around melanocytes, leading to melanocyte degeneration and pigmentation. On the other hand, in the early stages of the illness, skin with vitiligo was observed to have a substantial invasion of CD11c+ CXCL12+ dendritic cells (DCs) (13).
Upon comparing the serum level of CXCL13 among the different clinical patterns in the current study, the highest level was significantly (P = 0.043) recorded among patients manifesting a combination of vulgaris and acrofacial patterns.
In this study, there was a positive correlation between the severity of vitiligo, evaluated by VES score 13.99 (± 27.35), and serum CXCL13 level, which was statistically non-significant. Moreover, there was no statistically significant relationship between the existence of disease activity during the previous 6 weeks, assessed by the VIDA score, and serum CXCL13 levels. Bao et al. (14) discovered, in contrast to the current study’s findings, a substantial positive correlation between the blood level of CXCL13 and SLE activity. The importance of CXCL13 in the occurrence of numerous autoimmune dermatological illnesses, such as psoriasis and SLE, was also underlined by Pan et al. (5) in their study. They concentrated on the role of the CXCL13/CXCR5 axis in the development of these illnesses.
5.1. Conclusions
The CXCL13 serum level was higher in vitiligo patients than in healthy controls, suggesting that it could have a role in the etiopathogenesis of vitiligo. It is recommended to perform similar studies with a larger sample size and different ethnic groups and assess tissue expression of CXCL13 in vitiliginous skin.