1. Introduction
Sarcoidosis is considered a chronic disorder characterized by the presence of multiple granules affecting different body systems and tissues. These granules are histologically characterized by non-caseous granulomatous inflammation (1-5). This granulomatous disease is commonly identified in the pulmonary form, accounting for about 90% of cases. This disorder is also known to involve the respiratory system, lymphatic system, heart, eyes, nervous system, liver, as well as the renal and endocrine systems (6-10). The global average incidence of sarcoidosis has been estimated to be 16.5 to 19 cases per 100,000 population annually (5, 9, 11). Sarcoidosis is more prevalent in the USA, especially in people of African-American descent (35/100,000) (5, 9, 11). Although the prevalence of sarcoidosis is not known in Brazil, it has been estimated to be lower than 10 cases per 100,000 population (9).
The etiology of sarcoidosis is still unknown, but previous reports state that sarcoidosis may develop following the exposure of genetically susceptible individuals to specific environmental antigens and agents (8-12). The most common agents suggested to be involved in the etiology of sarcoidosis can be divided into three groups: (A) Infectious agents [such as viruses (herpes viruses, Epstein–Barr virus, retroviruses, coxsackie B virus, cytomegalovirus) and bacteria (Borrelia burgdorferi, Propionibacterium acnes, Mycoplasma. Mycobacterium tuberculosis, and other mycobacteria)]; (B) inorganic agents, for example, Aluminum, Zirconium, and Talc; and (C) organic, such as Pine tree pollen and Clay (8-12). Exposure to the above-described antigens triggers an inflammatory response, which is characterized by the presence of large numbers of activated macrophages and CD4+ T helper lymphocytes. These activated cells continuously produce specific cytokines, promoting the Th1-type immune response (8-10, 12).
Only 25% of sarcoidosis cases present with cutaneous manifestations, and it is rare in the head and neck regions, where it comprises about 10% to 15% of all cases (1, 5, 9, 11). Cutaneous lesions can range from red nodules in erythema nodosum to subcutaneous nodules, macules, and papules, sometimes presenting in scars as well. In a Brazilian study on cutaneous sarcoidosis, only 72 cases were identified in a tertiary hospital over 25 years, most of which were diagnosed in white females who were in the fifth decade of their lives. In 74% of these patients, cutaneous papules and plaques were the first manifestations (9).
Oral tissues are hardly involved, but in the literature, there are reports of the involvement of maxillary bones, the palate, buccal mucosa, the tongue, the floor of the mouth, and lips (7). In this region, such lesions may be the first or the only manifestation, ranging from single to multiple depositions. These lesions are usually painless nodules manifesting as ulcerations or papillary eruptions (8-10). These depositions are usually fixed in the underlying tissue, which is a characteristic of firm and asymptomatic submucosal masses (8-10). Accordingly, in a review published in 2016, less than 20 cases had been reported in the literature describing the isolated manifestation of sarcoidosis in oral tissues, most of which were identified in Caucasian female patients with a mean age of 39 years (5). The chief complaint was asymptomatic swelling, and pain was reported in less than 15% of the reviewed cases (5).
Histopathological examination must rule out other granulomatous diseases (11). In positive cases, non-necrotizing granulomas may be observed consisting of mononuclear histiocytes and giant cells surrounded by lymphocytes. Following histopathology examination, it is necessary to systematically investigate the patient to confirm the diagnosis, including complementary examinations such as posteroanterior chest radiography, spirometry, plethysmography, blood tests (hemogram and serological and biochemical tests), electrocardiography, urine analysis, and ophthalmology assessment (12). Considering that sarcoidosis is a very uncommon disease in the Brazilian population, the objective of this paper was to describe a very unusual case of sarcoidosis primarily manifesting with oral symptoms.
2. Case Presentation
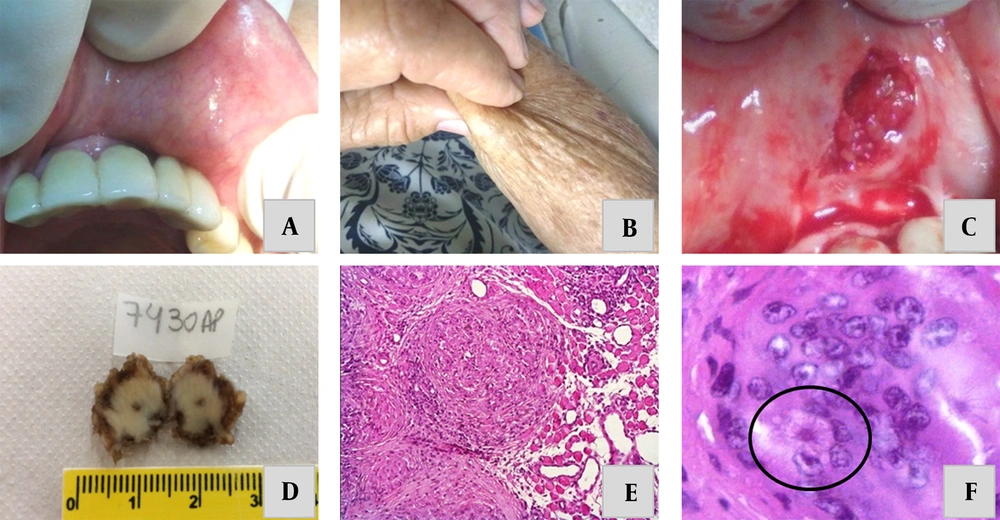
An 85-year-old, nonsmoking, white woman was referred to the Department of Stomatology of the University of Uberaba in 2019, complaining of a three-month-long persistently growing nodular lesion on the upper lip. During the anamnesis, the patient reported suffering from hypertension under the control of 50 mg of daily consumption of losartan potassium + 12.5 mg of hydrochlorothiazide. In the first appointment, all vital signs were within normal limits. Extraoral examination showed a slight volumetric increase in the left upper lip, and intraoral examination revealed a small nodular tissue. The lesion was asymptomatic and localized in the submucosa, showing a consistent and well-defined form, which was attached to underlining tissues. The lesion was measured approximately 1.5 cm in its largest diameter (Figure 1A). A similar but smaller nodule was also found on the left cheek and the right arm (Figure 1B). Cervical lymph nodes were not involved, and there was no prior diagnosis of neoplasia or other granulomatous infectious diseases. Moreover, there was no family history of sarcoidosis and no exposure to potential environmental and/or occupational triggering agents.
A, clinical presentation: Asymptomatic firm nodules beneath the upper lip. B, asymptomatic firm nodules on the right arm. C, excisional biopsy of the lesion. D, macroscopic appearance of the surgically removed specimen. E, histopathological analysis showing a non-infectious granuloma composing lobular aggregates of epithelioid histiocytes and Langhans giant cells. F, asteroid bodies inside a multinucleated Langhans giant cell.
An excisional biopsy was performed to clarify the benign nature of the lesion. The macroscopic appearance of the lesion showed a well-circumscribed specimen (Figure 1C). After suturing, the patient was released, and the biopsied tissue was sent for histopathological analysis (Figure 1D). One week after the surgery, the incised area showed obvious clinical signs of recovery, so the sutures were removed. Histopathologic examination showed fragments of oral mucosa with striated muscle bundles, adipocytes and ducts/acini of minor salivary glands, and a dense connective tissue exhibiting compact and lobular aggregates of epithelioid histiocytes (Figure 1E). On the margin of the histiocytic clusters, lymphocytes and giant Langerhans cells were seen. In addition, in focal granulomatous areas, some asteroid bodies (starry inclusions) could be visualized (Figure 1F). Warthin-Starry, Fite, and PAS special staining ruled out the presence of mycobacteria and fungal infections. Moreover, no pigmented, soluble, or polarizable foreign bodies were seen in the availed tissue.
Complementary tests were performed to confirm the possible diagnosis of sarcoidosis. Posteroanterior chest radiography did not indicate any significant changes in the lungs and heart. Blood tests revealed hypercalcemia (10.4 mEq/L) and high serum levels of angiotensin-converting enzyme (53.9 U/L) and alkaline phosphatase (189.3 U/L). Moreover, hematological, biochemical, serological, liver, and renal functional tests, urine analysis, pulmonary functional tests, electrocardiography, ophthalmological assessments, and the tuberculin skin test retrieved no remarkably deviated results.
The definitive diagnosis was sarcoidosis with oral manifestation, and the patient was brought to the attention of a medical service. No specific treatment was administered as the patient was asymptomatic, and there was no systemic involvement. Four years after the excisional biopsy, the surgery area healed uneventfully. It was observed that the nodules in the cheek and lips areas had the same initial characteristics and sizes. The patient remains under regular follow-up to monitor for the possible return of symptoms and complications.
3. Discussion
Sarcoidosis is characterized by the formation of non-caseating granulomas. It is mostly diagnosed in middle-aged adults as infiltrations/swellings in lymph nodes, lungs, eyes, and skin. Multiple tissues and organs can be involved at the same time. The diagnosis requires a combination of compatible clinical, histological (i.e., the presence of noncaseating granulomatous inflammation), hematological, and radiological findings (12). These granulomas often contain stellate inclusions, known as asteroid bodies (fragments of collagen), or laminated basophilic calcifications, known as Schaumann bodies (degenerative lysosomes) (3).
For the diagnosis of sarcoidosis, a compatible clinical picture must be integrated with the presence of noncaseating granulomatous inflammation in histopathology, and other disorders with similar signs and symptoms should be excluded. The most suitable place for performing a biopsy must be defined. Transbronchial lung has been recommended as the most suitable site for biopsy, requiring gaining 4 to 5 tissue fragments in a single attempt. When it is not possible to confirm a diagnosis with transbronchial biopsy, and there is no other accessible site for biopsy, it is recommended to obtain a lung biopsy by surgery in patients identified with lesions on chest radiography or computed tomography of the lung (12). Video thoracoscopic mediastinoscopy or open biopsy can also be performed, which is the first priority as it is associated with a lower rate of complications. These procedures deliver a diagnostic yield above 90% (12). However, biopsies of other sites, such as the skin, lips, lymph nodes, or granulomatous scars, can help reach the final diagnosis. In this way, biopsies of oral tissues appear to be an excellent diagnostic source since they are easily accessible by surgery, quickly heal, and show a very low rate of complications (1, 2, 4, 5).
The diagnostic approach for patients with sarcoidosis should achieve the following objectives: (1) Providing histopathological evidence of non-infectious granulomatous inflammation, (2) determining the level and severity of organ deposition, (3) evaluating whether the disease is stable or progressing, and (4) determining if therapy must be delivered (12). Following the histological confirmation of non-caseating granulomas, radiological examination, and blood/serum testing are recommended for all patients. Posteroanterior chest radiography, CT scan, or 67 Ga lung scan are indicated to evaluate the involvement of the heart, lung, and mediastinal lymph nodes (12). Blood/serum tests should be performed to detect derangements in the serum levels of calcium, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, creatinine, and blood urea, as well as angiotensin-converting enzyme (12).
In our patient, posteroanterior chest radiography and mediastinal CT scanning ruled out the presence of cardiac, pulmonary, and/or lymph nodal depositions. However, elevated serum levels of calcium, angiotensin-converting enzyme, and alkaline phosphatase, as biochemical markers of active sarcoidosis (12-14), confirmed our diagnosis. Hypercalcemia, which was also seen in our patient, occurs mainly due to the production of active vitamin D3 mediated by the 1-α-hydroxylase enzyme produced mainly by the macrophages existing in granulomas (12).
According to some authors, sarcoidosis is more prevalent in African Americans and North Europeans (11). In a study carried out in Brazil by Torquato et al., a higher prevalence was observed in white patients (61%). In Brazil, it is estimated that the prevalence of sarcoidosis is less than 10/100,000 population (9). In the United States, the annual incidence of sarcoidosis has been noted as 35.5/100,000 in blacks and 10.9/100,000 in whites (5, 9, 11). So, this condition seems to be extremely rare in Brazilians; however, an increase in its incidence has been reported in recent decades (13) with a predilection for young (25 and 35 years) and middle-aged (44 - 55 years) women (14). African-American and Northern European women are far more affected by this condition (11). Among Brazilians and those with oral manifestations, the disease mostly affects white/Caucasians (4, 5, 9). In our case, the patient shared similar clinicopathological features regarding gender and ethnicity; however, she was not in a typical age group in which the disease is usually found.
Depositions in thoracic (e.g., hilar, cardiac, and pleural) lymph nodes are described in the majority of cases, but other organs can also be affected (15). A cohort study conducted in the United States showed that in 50% of patients, the disease was localized to one area, mostly to the chest, and the involvement of extra-thoracic organs (i.e., the nasopharynx, peripheral lymph nodes, liver, skin, salivary glands, and peripheral and central nervous systems) was infrequent. The other half of patients presented lesions in two (30%), three (13%), four (5%), and five or more (2%) organs (16). In our patient, there were only 2 affected sites: The cheek and the upper lip, corroborating the findings of previous studies. Only 30% of patients with isolated skin lesions will show systemic involvement within 1 month to up to 1 year (17). After careful examination, our patient’s disease has not yet progressed to a systemic form after 4 years of follow-up.
Sarcoidosis was first described by Jonathan Hutchinson in 1875. However, the term sarcoidosis (the Greek term for "condition like flesh”) was only utilized 14 years later (3). In 1942, Schroff and collaborators were the first to report the involvement of the oral mucosa in sarcoidosis. Since then, only a few studies have been published describing the involvement of the oral region in sarcoidosis, mostly affecting gnathic bones, the cheek, gingiva, palate, floor of the mouth, and tongue. These lesions have been most commonly described as asymptomatic swellings with long-term evolution. Excluding the involvement of salivary glands and lymph nodes, clinically evident oral sarcoidosis is very uncommon (3), as herein reported.
Histologically, sarcoidosis was characterized by the presence of non-necrotizing granulomas in oral cavity lesions. Other diseases must be excluded before confirming the diagnosis, such as tuberculosis, Crohn’s disease, deep fungal infections, reactions to foreign bodies, or granulomatosis with polyangiitis. Special staining of tissue biopsies, as well as serological analyses and the tuberculin skin test, excluded any infectious diseases in our patient, favoring the final diagnosis of sarcoidosis.
There is no definitive therapeutic protocol for sarcoidosis, and some studies suggest just observation. Patients may benefit from surgical removal with or without radiotherapy. Systemic and local glucocorticoids, immunomodulators, and/or immunosuppressive agents seem to be the treatments of choice in some patients, requiring monitoring for the recovery or possible return of symptoms during treatment and after the discontinuation of these agents (12). More studies should be performed to conclude the effects of TNF-alpha antagonists, such as etanercet, infliximab, pentoxifylline, and thalidomide. Our patient did not receive glucocorticoids because she had no oral or systemic symptoms. She currently remains under follow-up to monitor any changes in her clinical condition.
The clinical evolution of sarcoidosis is still somewhat uncertain, but in half of the cases, the disease regresses and disappears spontaneously within 2 to 5 years after the onset of signs and symptoms. After this period, remissions/regressions was not expected to happen (12). The prognosis is usually good, and mortality due to cardiac, pulmonary, or central nervous system complications is expected in about 10% of the cases.
Overall, multidimensional professional evaluation is of paramount importance for the treatment of patients with sarcoidosis. The choice of treatment should be according to the possibility of spontaneous resolution and the possible side effects of prolonged use of medications like corticosteroids, methotrexate, azathioprine, chlorambucil, chloroquine, and cyclophosphamide (18). In conclusion, sarcoidosis can manifest in several ways, so the diagnostic process needs to be thorough and comprehensive to confirm the disease. In addition, patients should be periodically monitored to check disease progression.