1. Background
Eosinophils are a type of white blood cells (WBCs) constituting 1 - 5% of total leucocytes (1). Their presence in some tissues, such as lymphoid organs, the gastrointestinal tract, and lungs, is a normal finding, but homing of the skin by eosinophils is a pathologic phenomenon (2). An increase in the absolute eosinophil count (AEC) occurs in a plethora of pruritic-allergic, infective, and inflammatory dermatoses. Upon activation by cytokines such as interleukin-5, eosinophils serve as the prime mediators of pruritus and tissue damage through their ability to stimulate neuronal cells and release proteins such as eosinophilic cationic protein (ECP), eosinophil-derived neurotoxin (EDN) from their granules, and others such as substance P, vasoactive intestinal peptide, brain-derived neurotrophic factor, neurotrophin-3, nerve growth factor, and cytokines such as interleukins-4, 13, and 31 (3). IL-31 is the major mediator of itches in atopic dermatitis, prurigo nodularis, and dermatomyositis. In cutaneous T-cell lymphoma, eosinophilic infiltration correlates with itch severity (3). Blood eosinophilia, with or without tissue eosinophilia, can be the first alarming marker indicating either the onset, progression, or severity of pruritic dermatosis (4). Common causes of peripheral eosinophilia include urticaria, atopic dermatitis, drug reactions, parasitic infestations, vesiculobullous diseases, malignancies (e.g., lymphomas), and causes of metabolic pruritus (3). Absolute eosinophil count is considered a predictor of disease severity because the count of eosinophils correlates with tissue damage through the release of various inflammatory mediators (1, 3, 4).
The subjective impact of pruritus on quality of life (QoL) has been assessed in a multitude of dermatoses, such as atopic dermatitis, psoriasis, urticaria, and neurodermatitis (5-7). The dermatology life quality index (DLQI) is one of such subjective indicators measured by a questionnaire to help assess the psychosocial impact of dermatosis on the patient’s life and also predict the ‘perceived’ severity of the disease burden (8). Previous studies have noted a correlation between the severity of pruritic dermatoses and AEC (1, 4), as well as between DLQI and the subjective severity of pruritus (5-8).
This study aimed at investigating the potential direct association between AEC (an objective parameter of disease severity) and the DLQI score (a subjective marker of disease severity). Existing literature scrutinizing such a correlation is scarce, so this study may help develop new prognostic and therapeutic paradigms for predicting the severity of tissue damage and considering anti-eosinophil agents as potential therapeutic modalities at the outset of pruritic dermatoses based upon a well-designed QoL questionnaire.
2. Objectives
To determine if there exists a direct correlation between AEC and DLQI in patients diagnosed with different pruritic dermatoses of varied etiology.
3. Methods
This cross-sectional observational-correlational study was carried out in the Department of Dermatology of a tertiary governmental health care center affiliated with a medical college in Maharashtra, a state situated in Western India, over a period of six months from November 2020 to April 2021. The study was initiated after obtaining ethical approval from the institutional ethics committee. The study participants included both urban and rural patients. All male and female patients of the age group of 16 to 65 years visiting the dermatology outpatient clinic during the mentioned period with the diagnoses of various pruritic-infective, allergic, inflammatory, and malignant dermatoses were enrolled if they were willing to sign an informed consent form for participation. The selection of the participants in this study was through purposive/convenience sampling. Patients currently under treatment with drugs with potential effects on AEC (e.g., systemic anti-histamines/corticosteroids/dapsone, etc.) or on topical treatments within one month prior to enrolment were excluded. A detailed history was obtained regarding age, sex, occupation, drug history, disease history, and duration of pruritus. The dermatological diagnosis was made based on clinical grounds and confirmed by histopathology when required. A predesigned validated questionnaire (i.e., DLQI) was utilized to assess the impact of pruritus on QoL in patients with various diseases (8) after being translated into the vernacular (Marathi/Hindi) language. Patients unable to fill out the questionnaire were interviewed, and their responses were noted. Patients were categorized based on their dermatological diagnoses under various categories, including infectious, inflammatory, allergic, metabolic-related, pregnancy-associated, and drug-induced dermatoses.
The DLQI questionnaire is focused on the disease and its impact on QoL in the past week. The minimum score is 0, and the maximum score is 30, with higher scores indicating more impaired QoL. The scale contains a total of 10 questions, and each response is scored from a minimum score of 0 (no complaints) to 3 (serious complaints). The questionnaire has to be answered within two minutes. After calculating the total score, the impact of pruritus due to a particular disease on QoL was graded as 0-1: No impact on the patient’s life, 2 - 5: Minimal impact, 6 - 10: Moderate impact, 11 - 20: Profound impact, 21 - 30: Extremely profound impact.
After calculating the DLQI score, whole blood samples were collected from patients to determine AEC, which was graded as: (1) mild eosinophilia: 500 - 1000 eosinophils/µL, moderate eosinophilia: 1000 to 1500 eosinophils/µL, severe eosinophilia: > 1500 eosinophils/µL.
After categorizing patients with pruritic dermatoses based on the results of DLQI and AEC, the correlation between the two parameters was analyzed. Qualitative data were represented using frequency and percentage. The association between qualitative variables was assessed by the chi-square test. Quantitative data were presented using mean +/- standard deviation and median. A p-value of < 0.05 was considered statistically significant. Pearson’s correlation coefficient was calculated to determine if there was any correlation between AEC and DLQI. Other variables like demographics (age, gender) and disease-related parameters (etiology, duration, etc.) were also analyzed. SPSS version 21.0 was used for data analysis, and Microsoft Excel 2010 for drawing graphs.
4. Results
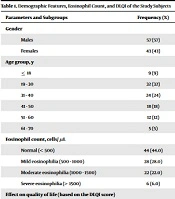
Over the 6-month study period, 100 patients with various pruritic dermatoses were enrolled, with a slight male preponderance (57% males versus 43% females). Most patients were in the third (32%) and fourth (24%) decades of life. Only 9% of the cases were below 18 years, and 5% were aged above 60 years (Table 1). The average age of the participants was 35.9 ± 13.4 years (Table 2).
| Parameters and Subgroups | Frequency (%) |
|---|---|
| Gender | |
| Males | 57 (57) |
| Females | 43 (43) |
| Age group, y | |
| ≤ 18 | 9 (9) |
| 19 - 30 | 32 (32) |
| 31 - 40 | 24 (24) |
| 41 - 50 | 18 (18) |
| 51 - 60 | 12 (12) |
| 61 - 70 | 5 (5) |
| Eosinophil count, cells/ µL | |
| Normal (< 500) | 44 (44.0) |
| Mild eosinophilia (500 - 1000) | 28 (28.0) |
| Moderate eosinophilia (1000 - 1500) | 22 (22.0) |
| Severe eosinophilia (> 1500) | 6 (6.0) |
| Effect on quality of life (based on the DLQI score) | |
| Extremely large | 12 (12.0) |
| Moderate | 35 (34.0) |
| No effect | 5 (5.0) |
| Small | 11 (8.0) |
| Very large | 37 (37.0) |
Demographic Features, Eosinophil Count, and DLQI of the Study Subjects
| Parameters | No. | Range | Minimum | Maximum | Mean ± SD | Skewness |
|---|---|---|---|---|---|---|
| Age, y | 100 | 49 | 16 | 65 | 35.9 ± 13.4 | 0.447 |
| Absolute eosinophil count, cells/µL | 100 | 2307 | 63 | 2370 | 740.2 ± 487.0 | 1.034 |
| DLQI score | 100 | 27 | 0 | 27 | 11.3 ± 6.0 | 0.511 |
Descriptive Statistics of Age, AEC, and DLQI
Most patients had inflammatory diseases (26%) [psoriasis: 5%, contact dermatitis: 5%, atopic dermatitis: 4%, photodermatitis: 3%, seborrheic dermatitis: 2%, lichen planus: 2%, neurodermatitis: 2%, polymorphic light eruption: 1%, Churg Strauss syndrome: 1%, and eosinophilic pustular folliculitis: 1%]. Infections constituted 16% of the total cases, including dermatophytosis (10%), candidial intertrigo (2%), HIV-related pruritic papular eruption (2%), and furunculosis (2%). Patients with chronic (> 6 weeks) urticaria constituted 8% of the cases, while the rest (6% of the patients) had an acute presentation. Dermatoses with underlying metabolic disturbances (10%) were due to chronic kidney disease (3%), hypothyroidism (3%), chronic liver disease (2%), and uncontrolled diabetes (2%). Among patients with drug-triggered reactions (9%), the patterns encountered were drug-related eosinophilia and systemic symptoms (DRESS) (2%), exanthematous eruptions (2%), anti-tubercular treatment-induced eczematous eruption (1%), Stevens-Johnson syndrome (1%), anti-retroviral therapy-induced pruritus (1%), imatinib-induced psoriasiform eruption (1%), and acute generalized exanthematous pustulosis (1%). Infestations comprised 9% of the total cases, including scabies (4%) and pediculosis (2%) leading the list, whereas there were single cases of cutaneous larva migrans (1%), demodex folliculitis (1%), and arthropod bite reaction (1%). Amongst 5% of patients with neoplasia, 2 cases had non-Hodgkin’s lymphoma (2%), followed by single cases with Hodgkin's lymphoma, leukemia cutis, and polycythemia vera. Pregnancy-related pruritic dermatoses consisted of 5% of the total cases, encompassing prurigo of pregnancy (2%), pruritic urticarial papules and plaques of pregnancy (1%), early onset atopic dermatitis of pregnancy (1%), and cholestasis of pregnancy (1%). Vesiculobullous diseases were seen among 4% of the cases, including bullous pemphigoid (2%), pemphigus vegetans (1%), and dermatitis herpetiformis (1%). There were single cases of each psycho-cutaneous disorder (functional itch disorder) and immunodeficiency (Job’s syndrome) (Table 3).
| Underlying Causes | No. | AEC | Grouped Median | Range | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Drug reaction | 9 | 263.0 | 426.5 | 1274 | 1198.0 |
| Immunodeficiency | 1 | 315.2 | NA | 2370 | 0.0 |
| Infections | 16 | 405.4 | 150.4 | 395 | 608.0 |
| Infestation | 9 | 580.2 | 361.3 | 656 | 1067.0 |
| Inflammatory conditions | 26 | 626.1 | 359.2 | 478 | 1107.0 |
| Metabolic disturbances | 10 | 654.6 | 153.8 | 294 | 537.0 |
| Neoplasia | 5 | 740.2 | 548.3 | 1566 | 1522.0 |
| Pregnancy dermatosis | 5 | 927.3 | 297.0 | 480 | 768.0 |
| Psychocutaneous | 1 | 984.5 | NA | 263 | 0.0 |
| Urticaria | 14 | 1261.6 | 301.4 | 1016 | 1010.0 |
| Vesiculobullous | 4 | 1565.8 | 454.9 | 945 | 910.0 |
| Total | 100 | 2370.0 | 487.0 | 600.0 | 2307.0 |
Descriptive Statistics of AEC in Various Subgroups
Mild eosinophilia (500 - 1000 cells/µL) was observed in 28% of the cases, moderate eosinophilia (1000 - 1500 cells/µL) in 22%, and severe eosinophilia (> 1500 cells/µL) in 6% (Table 1).
Among infective dermatoses, 80% of patients with dermatophytic infections had normal eosinophil count, and the remaining 20% showed mild eosinophilia. Over half (55%) of patients with infestations (mainly scabies, pediculosis, and demodex folliculitis-single) showed mild eosinophilia, and 33% of these patients revealed a normal eosinophil count. Upon those with inflammatory dermatoses3 out of 4 (75%), patients with atopic dermatitis showed moderate eosinophilia, whereas all 5 patients with psoriasis had normal AEC or mild eosinophilia. Irritant contact dermatitis and seborrhoeic dermatitis were associated with normal eosinophil counts, whereas 33% of cases with allergic contact dermatitis showed moderate eosinophilia.
Overall, moderate eosinophilia was observed in 22% of all patients, of whom the major proportion belonged to urticaria (50%) and drug reactions (44%). Among 14 patients with urticaria, 8 patients had chronic disease, and the remaining 6 individuals had acute urticaria. Moderate eosinophilia was found in 50% of patients with chronic urticaria, whereas in those with acute presentation, eosinophil counts were either normal to slightly elevated. Only 6% of all subjects showed severely raised eosinophil counts, of whom 3 patients (50%) had neoplasia (non-Hodgkin lymphoma, Hodgkin lymphoma, and cutaneous T-cell lymphoma/leukemia); two cases (33%) had adverse drug reactions (including DRESS syndrome), and 1 patient had immunodeficiency disorder. Regarding drug reactions, both patients with DRESS syndrome revealed severe eosinophilia; 44% of them showed moderate eosinophilia due to exanthematous eruption, eczematous rash, Stevens-Johnson syndrome, and acute generalized exanthematous pustulosis.
The analysis of patients with vesiculobullous disease revealed that 50% of these individuals had moderate eosinophilia (bullous pemphigoid), 25% showed mild eosinophilia (pemphigus vegetans), and 25% (dermatitis herpetiformis) had normal counts. Finally, 44% of all cases showed normal eosinophil counts, comprising patients with infections and infestations (64%), metabolic diseases (80%), pregnancy dermatoses (60%), and inflammatory diseases (50%).
The mean AEC was found to be 740.2 ± 487cells/µL (Table 2), with a median count of 800 cells/µL. The direction of outliers caused positive skewness (a longer tail on the right side), deviating from the perfect bell-shaped normal distribution. Therefore, there were more points (for both AEC and DLQI) on the left side of the distribution graph and only a few points on the right side, placing the mean on the right side of the median. Average AEC values were greater than the median (i.e., 800 cells/µL) in pregnancy dermatoses (927.3 ± 297 cells/µL), psycho-cutaneous (984.5 cells/µL), urticaria (1261.6 ± 301.4 cells/µL), and vesiculobullous (1565.8 ± 487 cells/µL). Average AEC values in other conditions were lower than the median (i.e., 800 cells/µL) (Table 3). Significantly higher numbers of subjects in the drug reaction, immunodeficiency, infestation, neoplasia, urticaria, and vesiculobullous categories had AECs greater than the median (P < 0.0001, Table 4).
| Disease Category | Absolute Eosinophil Count-Cells/µL | P-Value | DLQI | P-Value | ||
|---|---|---|---|---|---|---|
| No. of Patients > Median | No. of Patients ≤ Median | > Median | ≤ Median | |||
| Drug reactions | 8 | 1 | < 0.0001 | 6 | 3 | < 0.0001 |
| Immunodeficiency | 1 | 0 | 1 | 0 | ||
| Infections | 2 | 14 | 0 | 16 | ||
| Infestation | 5 | 4 | 2 | 7 | ||
| Inflammatory conditions | 11 | 15 | 17 | 9 | ||
| Metabolic disturbance | 0 | 10 | 3 | 7 | ||
| Neoplasia | 5 | 0 | 5 | 0 | ||
| Pregnancy dermatosis | 1 | 4 | 1 | 4 | ||
| Psychocutaneous | 0 | 1 | 1 | 0 | ||
| Urticaria | 12 | 2 | 9 | 5 | ||
| Vesiculobullous | 3 | 1 | 4 | 0 | ||
Distribution of Patients According to the Medians of AEC and DLQI Score
Based on the DLQI score, the impact of pruritic dermatoses of various causes on QoL was found to be very large in 37% of cases and extremely large among 12% of patients. Quality of life was unaffected in 5% of the cases. The number of subjects with DLQI scores greater than the median was significantly higher among patients with drug reactions, inflammatory conditions, neoplastic conditions, and urticaria (P < 0.0001, Table 4). There was a significant strong positive correlation between AEC and DLQI regarding all disease categories (Pearson’s correlation coefficient [r] =0.649, P < 0.0001, Table 5). A significant strong positive correlation was also noted between AEC and DLQI in patients with urticaria (r = 0.699), patients aged ≤ 50 (r = 0.633) and > 50 (r = 0.649) years old, men (r = 0.649), and women (r = 0.653, Table 5). There was also a significant positive correlation between AEC and DLQI in the drug reaction subgroup (n = 9) (r = 0.739, P = 0.023). In other disease categories, AEC and DLQI did not demonstrate any correlation. Age was positively correlated with DLQI, indicating poorer QoL among older individuals (r = 0.253, P = 0.011). There was no correlation between age and AEC.
| Disease Category | No. | Pearson Correlation Coefficient (R) | P-Value |
|---|---|---|---|
| Drug reactions | 9 | 0.739 | 0.023 |
| Infections | 16 | 0.228 | 0.395 |
| Infestation | 9 | 0.621 | 0.074 |
| Inflammatory conditions | 26 | 0.201 | 0.234 |
| Metabolic disturbance | 10 | 0.687 | 0.028 |
| Neoplasia | 5 | 0.237 | 0.701 |
| Pregnancy dermatosis | 5 | 0.151 | 0.808 |
| Urticaria | 14 | 0.669 | 0.009 |
| Vesiculobullous | 4 | 0.276 | 0.724 |
| Total | 100 | 0.649 | < 0.0001 |
| Age, y | |||
| ≤ 50 | 83 | 0.633 | < 0.0001 |
| > 50 | 17 | 0.696 | 0.002 |
| Gender | |||
| Male | 57 | 0.649 | < 0.0001 |
| Female | 43 | 0.653 | < 0.0001 |
| Correlation of Age with DLQI | |||
| Age | 100 | 0.253 | 0.011 |
| Correlation of Age with AEC | |||
| Age | 100 | 0.086 | 0.395 |
Correlation Between AEC and DLQI in Each Disease Category, Age Group, and Gender
5. Discussion
Eosinophils were first described by Paul Ehrlich in 1879 (9). Although the presence of eosinophils in the skin can be benign and transient, as in erythema toxicum neonatorum (10) and incontinentia pigmenti, it may cause profound damage as well, as in hyper-eosinophilic syndrome (11). In our study, inflammatory dermatoses were found to be related to infective causes and urticaria as the major etiologies for pruritic diseases encountered. Most patients with mild eosinophilia had infections or infestations (32%), followed by inflammatory dermatoses such as eczema and psoriasis (28%). Overall, moderate eosinophilia was observed in 22%, of which 50% were due to urticaria, 44% were secondary to drug reactions, and 6 % were related to vesiculobullous diseases. Most patients with severe eosinophilia had malignancies (50%), followed by DRESS syndrome and immunodeficiency (each in a single case).
In a study conducted by Radonjic-Hoesli et al. (4), a total of 453 patients (51.4% females; mean age: 58.4 ± 21.7 years) were included and categorized according to blood AEC and eosinophilia severity as severe: ≥ 1500 cells/µL (n = 87; 19.2%); moderate: 1000 to 1499 cells/µL (n = 73; 16.1%); and mild: 500 to 999 cells/ µL (n = 293; 64.7%). Most patients presented with chronic diseases (64.6%), generalized skin lesions (75.9%), and pruritus (88.1%). Statistical analyses revealed three patterns: (1) mild eosinophilia associated with localized skin disease, age < 50 years, history of atopy, and the diagnosis of eczema or infectious diseases; (2) moderate eosinophilia linked to generalized skin lesions, pruritus, age above 70 years, and autoimmune bullous disease; and (3) severe eosinophilia associated with hypereosinophilic syndromes, drug hypersensitivity, or malignant diseases.
In an Indian study conducted by Subramony et al. (1), mild-moderate eosinophilia was found in 98% of the cases, and only 2% of patients showed a remarkable increase in eosinophil counts. The most common disease categories encountered were urticaria (28%) and generalized pruritus (16%). In a study conducted on 158 patients by Burkhart, underlying causes were identified as localized neurodermatitis (48%), atopic dermatitis (39%), exudative neurodermatitis (6%), dermatitis herpetiformis (6%), and pemphigus vegetans (2%). They observed that 88% of the patients diagnosed with exudative neurodermatitis, 61% of those with atopic dermatitis, 55% of patients with dermatitis herpetiformis, and all pemphigus vegetans cases showed variable degrees of eosinophilia, but there was no report on the severity of eosinophilia in these conditions (12).
In a study conducted by Warlich et al. (5) to assess health-related QoL (HRQoL) in 510 patients (282 females; median age: 61.4 years) with chronic pruritus, a significant correlation was noted between DLQI and the visual analog scale (VAS) score, irrespective of the type of skin lesion. Overall, women had a lower HRQoL compared to men (females: 10.7 ± 6.7, males: 8.9 ± 6.7); however, the female gender was associated with worse QoL only in patients younger than 65 years old.
Our cross-sectional study was designed to assess the correlation between AEC (an objective indicator of disease severity) and DLQ (a perceived indicator of disease severity), finding a strong positive association between these parameters regarding all disease categories, as well as in the urticaria and drug reaction subgroups. This finding suggests that the primary tissue damage in these conditions can be attributed to eosinophils and directly translated into poor QoL. On the other hand, inflammatory dermatoses (like psoriasis) and neoplastic diseases also impaired QoL (i.e., higher DLQI scores), which was not proportional to the objective severity estimated by eosinophil count. Therefore, AEC can not be regarded as a primary marker of tissue damage in these conditions, reflecting that an increase in AEC in some cases may be secondary to other inflammatory mediators besides histamine (13). This finding is in accordance with the clinical observation suggesting that pruritus is often refractory to conventional antihistamines in most patients with inflammatory and neoplastic disorders.
The main strength of our study lies in its novel attempt to divulge the correlation between AEC and DLQI, opening a potential target (i.e., eosinophils) for therapeutic interventions and disease prognostication, wherein a subjective questionnaire (i.e., DLQI) would assist clinicians in gauging the ongoing tissue damage. The notable limitations are the cross-sectional study design and the small number of patients included under each disease category, precluding a statistical comparison between different disorders, which likely attenuated the power of the study.
5.1. Conclusions
A statistically significant correlation was observed between AEC (an objective disease severity marker) and DLQI (a subjective marker of disease burden) in patients suffering from various pruritic-allergic, infective, and inflammatory dermatoses. Thus, AEC can be regarded as a surrogate marker for true and perceived disease prognostication, as well as an objective equivalent of impaired QoL, holding promises as a potential hematological target for therapeutic interventions.