1. Background
Atopic dermatitis (AD) is a chronic recurring skin condition that affects up to 20% of children and adolescents worldwide (1) and has a prevalence of 13.4% among Malaysian children (2). The current primary pharmacological treatments for AD include emollients, topical corticosteroids (TCS), immunosuppressants, antimicrobial and antiseptic agents, antihistamines, and mast cell stabilizers (3).
The introduction of topical hydrocortisone in 1952 by Sulzberger and Witten marked a significant historical milestone, leading to the development of numerous new TCS with varying potencies. These advancements have made the treatment of various inflammatory skin disorders more effective and less time-consuming (4).
Topical corticosteroids have been associated with both local and systemic side effects, including cutaneous atrophy, telangiectasia, striae, steroid rosacea, perioral dermatitis, hypothalamic-pituitary-adrenal axis suppression, and skin infections (5). Many parents who are concerned about the potential long-term adverse effects of TCS tend to avoid using them, resulting in non-compliance with treatment and poor control of moderate and severe AD in children (5).
Mometasone furoate is a non-fluorinated synthetic glucocorticoid that is effective while having minimal side effects, such as skin atrophy (6). Studies have shown that mometasone cream provides rapid symptom relief in acute exacerbations of AD and is suitable for long-term maintenance use, with a longer period of remission compared to other corticosteroids (6). It was observed that mometasone cream reduced the severity of acute skin lesions, including vesicles, erosions, and itching, in a shorter time (2 - 3 days) compared to betamethasone dipropionate (4 – 5 days) (6).
Despite the proven efficacy of mometasone furoate in various clinical trials, there has been limited research on the maximum duration for continuous use before changes in skin thickness occur. One study by Kelly et al. investigated the local and systemic effects of mometasone furoate 0.1% cream over a 3-month continuous period in adolescents and adults aged at least 15 years (7). Mild signs of skin atrophy were only detected after 4 to 12 weeks of treatment with mometasone furoate 0.1% cream (7).
Guidelines from the National Institute for Health and Care Excellence (NICE) recommend that in children aged 12 months and older, potent TCS should not be used continuously for more than 14 days on the trunk and limbs, and it is strictly contraindicated for use on the face and neck (8). For infants younger than 12 months, the use of potent TCS should be closely monitored by a dermatologist (8). The Malaysian clinical practice guidelines for atopic eczema in 2018 suggest using the least potent but effective TCS in children, and while short courses of moderate to potent TCS can be used during acute flares, they do not specify the maximum duration of use (9).
However, concerns about potential local and systemic adverse effects often lead to parental non-compliance with TCS use in the treatment of AD and may result in mislabeling treatment failures.
2. Objectives
In this study, we aim to explore the atrophogenic effects associated with mometasone furoate topical therapy.
3. Methods
3.1. Study Design
We conducted a single-center, prospective cohort study involving children aged 6 to 12 years who were under the care of the Pediatric Dermatology Clinic at Hospital Tunku Azizah and had moderate to severe AD. This study received approval from the Medical Research and Ethics Committee, Ministry of Health, Malaysia (NMRR-20-2666-57115).
3.2. Eligibility Criteria
All children aged 6 to 12 years with moderate-to-severe AD who had lesions on the volar aspect of either forearm were assessed for eligibility and recruited into the study. Written informed consent was obtained from the parents or legal guardians of each participant.
3.3. Exclusion Criteria
Patients with underlying immunodeficiencies, inborn errors of metabolism, congenital syndromic disorders, intellectual impairment, co-existing psoriasis, or ongoing cutaneous infections were excluded from the study. Additionally, patients who had used mometasone furoate cream on their forearms or received systemic corticosteroids within the past 4 weeks were also excluded. Non-compliant patients who did not adhere to study instructions or attend study visits were excluded.
3.4. Procedure
All enrolled subjects were instructed to apply TCS over a 6-week period, with clinic reviews scheduled at baseline, 2 weeks, and 6 weeks. Mometasone furoate 0.1% cream was chosen as the topical corticosteroid due to its availability in Malaysia. Cream formulation was preferred over ointment, considering the tropical climate, as it typically leads to better compliance among children.
Mometasone furoate 0.1% cream was applied twice daily to the eczematous skin on the volar aspect of the forearm without occlusion, wet wraps, or dressing. Only moisturizers such as aqueous cream or aqueous cream with 25% glycerin were allowed to be used alongside mometasone cream during the 6-week study period. Other TCS, combinations, or alternative agents such as organic cream, mineral oil, olive oil, coconut oil, baby oil, traditional ointments, herbs, honey, or over-the-counter prescription creams were strictly prohibited during the study.
To ensure compliance, we provided written instructions to all participants regarding the timing and frequency of application. Video calls were conducted twice daily to directly observe the application of mometasone furoate 0.1% cream on the study area. Parents and caregivers were responsible for applying mometasone cream to the forearm lesions during each video call, ensuring that 1 fingertip unit was applied to each forearm. Participants' parents and caregivers also received phone calls 1 day before and on the day of their clinic reviews.
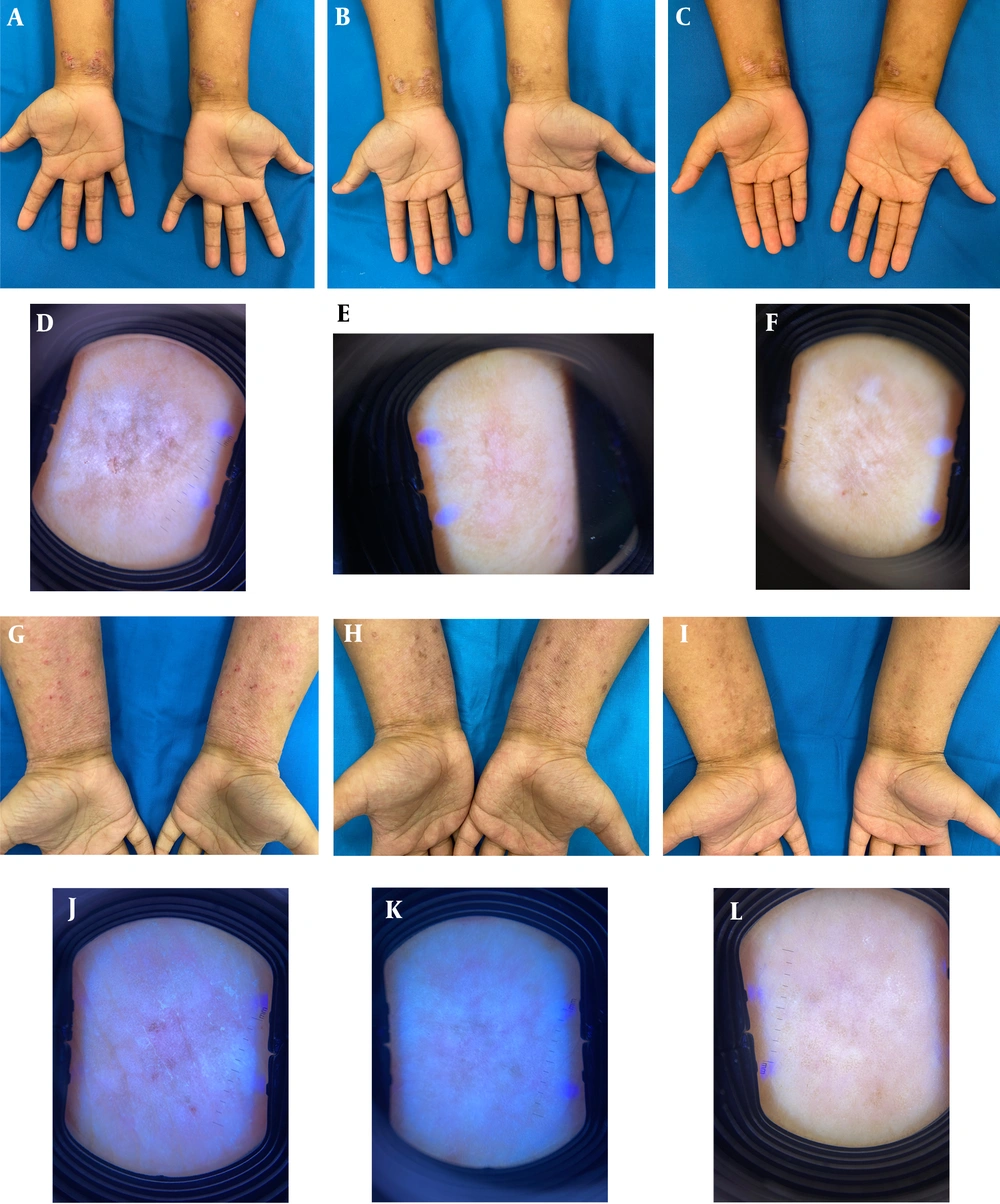
At each clinic visit, 2 pediatric dermatologists independently performed a dermoscopy on the volar aspect of the left forearm, 2 cm from the distal skin crease at the midline. This evaluation aimed to assess clinical signs of atrophy, including skin wrinkling, increased skin transparency, shininess, telangiectasia, bruising, and loss of skin markings (Table 1). The severity of skin atrophy was graded using a scoring system as follows: 0 = none or absent, 1 = mild, 2 = moderate (easily discernible), and 3 = severe or extensive (markedly evident). Any discrepancies between the 2 dermatologists were resolved through discussion or, if necessary, consultation with a third dermatologist. Erythema improvement, sleep loss, and itch severity at the study area were also evaluated during each clinic visit. Demographic and clinical data of the patients were recorded (Table 2).
| Study Period | Study Week | ||||||
|---|---|---|---|---|---|---|---|
| Treatment week | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Clinic visit number | 1 | 2 | 3 | ||||
| Clinic visit window, d | ±2 | ±2 | |||||
| Study enrolment | x | ||||||
| Clinical and medication review | x | x | x | ||||
| SCORAD a | x | x | x | ||||
| Itch scoring b | x | x | x | ||||
| Sleep loss scoring c | x | x | x | ||||
| Clinical scoring d | x | x | x | ||||
| Dermoscopy | x | x | x | ||||
| Ultrasonographic skin thickness measurement | x | x | x | ||||
| Treatment instruction e | x | x | x | x | x | x | x |
| Video calls f | x | x | x | x | x | x | x |
Timeframe of Follow-up Procedures Throughout the Study Period
| Case | Age (y) | Eczema Onset (y) | Baseline | Week 2 | Week 6 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCORAD a | Erythema b | Itch c | Sleep Loss d | Skin Atrophy e | Dermal Thickness f | SCORAD a | Erythema b | Itch c | Sleep Loss d | Skin Atrophy e | Dermal Thickness f | SCORAD a | Erythema b | Itch c | Sleep Loss d | Skin Atrophy e | Dermal Thickness f | |||
| 1 | 11.8 | 0.5 | 80.8 | 3 | 8 | 8 | No | 1.2 | 30.8 | 2 | 3 | 2 | No | 1.1 | 26.3 | 1 | 2 | 2 | No | 1.07 |
| 2 | 7.3 | 0.3 | 38.2 | 2 | 5 | 5 | No | 0.63 | 38.6 | 1 | 3 | 2 | No | 0.63 | 37.9 | 0 | 8 | 1 | No | 0.63 |
| 3 | 8.6 | 1.0 | 27 | 2 | 7 | 5 | No | 0.84 | 23.6 | 1 | 3 | 5 | No | 0.76 | 18.2 | 0 | 3 | 0 | No | 0.76 |
| 4 | 9.6 | 1.0 | 19 | 1 | 2 | 2 | No | 0.54 | 25.5 | 1 | 10 | 9 | No | 0.53 | 41.2 | 0 | 5 | 0 | No | 0.57 |
| 5 | 10.7 | 6.0 | 26 | 2 | 2 | 1 | No | 0.86 | 16.5 | 2 | 2 | 1 | No | 0.9 | 22.9 | 1 | 2 | 1 | No | 0.86 |
| 6 | 10.7 | 0.5 | 20 | 1 | 6 | 1 | No | 0.7 | 18.5 | 0 | 5 | 1 | No | 0.6 | 13.2 | 0 | 5 | 1 | No | 0.57 |
| 7 | 6.2 | 0.1 | 46.5 | 2 | 6 | 5 | No | 0.83 | 26.3 | 1 | 4 | 3 | No | 0.87 | 27.3 | 1 | 4 | 4 | No | 0.8 |
| 8 | 11.1 | 2.0 | 46.9 | 1 | 5 | 5 | No | 0.67 | 45.9 | 0 | 6 | 6 | No | 0.53 | 25.4 | 0 | 2 | 1 | No | 0.63 |
| 9 | 6.0 | 0.2 | 26 | 1 | 4 | 4 | No | 0.63 | 32 | 1 | 4 | 3 | No | 0.63 | 37 | 1 | 8 | 5 | No | 0.63 |
| 10 | 11.8 | 4.0 | 40.9 | 2 | 5 | 0 | No | 0.83 | 22.4 | 1 | 5 | 0 | No | 0.83 | 20 | 0 | 5 | 0 | No | 0.9 |
| 11 | 8.1 | 1.0 | 32.4 | 2 | 7 | 7 | No | 0.57 | 34.1 | 0 | 5 | 3 | No | 0.53 | 33.2 | 0 | 2 | 3 | No | 0.6 |
| 12 | 5.9 | 5.0 | 19 | 2 | 3 | 1 | No | 0.53 | 16.2 | 2 | 1 | 0 | No | 0.57 | 24.2 | 1 | 2 | 1 | No | 0.57 |
| 13 | 10.5 | 3.0 | 45.1 | 1 | 6 | 2 | No | 0.87 | 35.4 | 1 | 6 | 2 | No | 0.8 | 36.3 | 0 | 7 | 0 | No | 0.8 |
| 14 | 6.4 | 0.2 | 55.1 | 1 | 6 | 5 | No | 0.73 | 44.3 | 1 | 7 | 4 | No | 0.8 | 29.4 | 0 | 4 | 4 | No | 0.8 |
| 15 | 11.8 | 0.2 | 65.3 | 2 | 5 | 6 | No | 0.5 | 27.8 | 0 | 2 | 2 | No | 0.9 | 26.7 | 0 | 8 | 5 | No | 0.8 |
| 16 | 7.7 | 0.5 | 23.2 | 1 | 5 | 3 | No | 0.7 | 20.2 | 1 | 5 | 0 | No | 0.7 | 15.8 | 0 | 3 | 0 | No | 0.7 |
| 17 | 6.6 | 0.1 | 26.2 | 2 | 9 | 2 | No | 0.8 | 23.8 | 1 | 7 | 2 | No | 0.77 | 19.4 | 0 | 3 | 1 | No | 0.77 |
| 18 | 6.6 | 0.2 | 35.8 | 1 | 7 | 6 | No | 0.56 | 35 | 0 | 4 | 3 | No | 0.56 | 19.4 | 0 | 4 | 0 | No | 0.56 |
| 19 | 9.2 | 2.0 | 26.4 | 1 | 8 | 3 | No | 0.8 | 18.5 | 0 | 5 | 4 | No | 0.77 | 23.8 | 0 | 7 | 0 | No | 0.77 |
| 20 | 6.1 | 2.0 | 30.4 | 3 | 3 | 4 | No | 0.7 | 32.5 | 2 | 7 | 6 | No | 0.63 | 26.6 | 0 | 6 | 5 | No | 0.57 |
| 21 | 11.4 | 4.0 | 16 | 1 | 1 | 0 | No | 0.73 | 12.5 | 1 | 1 | 0 | No | 0.8 | 12.3 | 0 | 0 | 0 | No | 0.77 |
| 22 | 12.8 | 9.0 | 27.9 | 2 | 5 | 2 | No | 0.8 | 22.8 | 2 | 3 | 2 | No | 0.8 | 21.2 | 1 | 2 | 1 | No | 0.8 |
Clinical Characteristics of the Patients in Our Cohort
Skin thickness and dermal thickness in millimeters (mm) were measured by 2 radiologists using high-frequency ultrasound scans (GE S7XD Clear) with a linear probe (L18MHz) at 0, 2, and 6 weeks after the application of mometasone furoate cream. All measurements were taken on the volar aspect of the forearm, 2 centimeters from the distal skin crease at the midline, using the musculoskeletal (MSK) general ultrasound setting. Both the attending pediatric dermatologist and radiologist were blinded to the severity of AD, duration of mometasone use, and stage of the study visit during their assessments.
3.5. Statistical Analyses
The study's statistical power was calculated using G*Power v. 3.1.9.4. At baseline, all patients had moderate-to-severe AD. After 6 weeks of continuous mometasone cream 0.1% use, 6 out of 22 (27.3%) patients had mild disease, and 16 (73.7%) no longer had erythema. The study's power was determined to be 86%.
Data were analyzed using IBM SPSS v. 26 (SPSS Inc., Chicago, Illinois). Associations between categorical variables were assessed using Pearson's chi-squared test or Fisher's exact test. As all continuous variables had a non-normal distribution, they were described as median (interquartile range), and the Mann-Whitney U test was applied. A significance level of 0.05 was used for all tests.
4. Results
A total of 586 children with AD were screened, and 562 patients were excluded for various reasons: not having forearm lesions (n = 421), not requiring mometasone cream at the study area (n = 120), or having a cutaneous infection at the study area during the screening (n = 20). Twenty-four patients with moderate-to-severe AD were recruited for this study. Two patients dropped out—1 child developed a varicella-zoster infection during the study, and another patient missed the scheduled clinic visit and ultrasound appointment. Consequently, a total of 22 patients were included in the study analysis, with a male-to-female ratio of 1:1 and a median age at study recruitment of 8.9 years (range = 6.0 - 12.7) (Table 1). Our patient cohort had a median age at disease onset of 12 months (range = 1.2 - 108) and an AD duration of 7.1 years (range = 0.9 - 11.7). Thirteen patients had severe AD (59.1%), all of whom had tried systematic therapy for more than 4 weeks before study recruitment, while 9 patients had moderate AD (40.9%) and were only on TCS (Table 2). Systemic therapy included 8 patients on cyclosporin, 3 on methotrexate, and 2 on azathioprine. None of the patients in our cohort were on biologics, janus kinase (JAK) inhibitors, or systemic glucocorticoids. There were no discrepancies in the clinical scoring of the patients between the 2 assessors, except for patient 22, whose skin erythema score at the final clinic visit was given a 0 by the first assessor but a score of 1 by the second assessor. A third pediatric dermatologist was consulted to assess the level of erythema, and a score of 1 was assigned.
4.1. Atopic Dermatitis
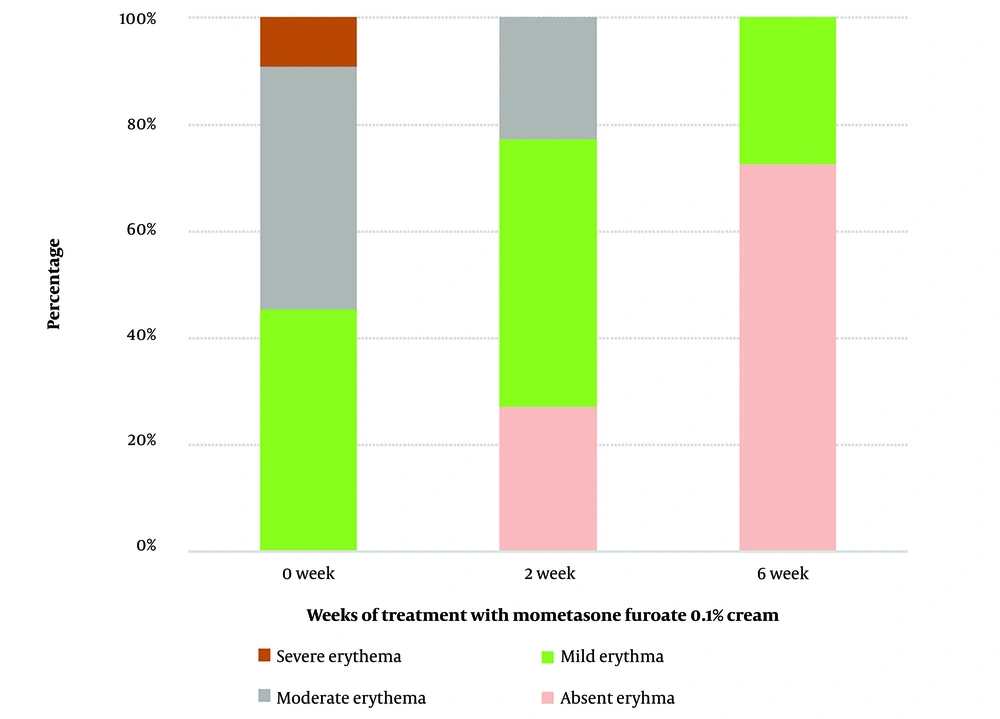
At baseline, 10 patients (45.5%) exhibited mild erythema, 10 patients (45.5%) had moderate erythema, and 2 patients (9.1%) displayed severe erythema on dermoscopy at the volar aspects of both forearms (Table 3). Dermoscopic examination at the 2-week follow-up revealed that 11 patients had mild erythema (50%), 5 showed moderate erythema (22.7%), and 6 patients had no clinically visible erythema at the study area (27.3%). At the end of the 6-week study period, only 6 patients had mild skin erythema (27.3%), while 16 patients had no clinical erythema (72.7%) (Figures 1 and 2). The reduction in skin erythema over the 6-week period of mometasone use was statistically significant, with a P-value of < 0.001 (Table 3).
| Variables | No. (%) or Median (Range) |
|---|---|
| Age at study recruitment, y | 8.9 (6.0 - 12.7) |
| Age of AD onset, y | 1.0 (0.1 - 9.0) |
| Duration of AD, y | 7.1 (0.9 - 11.7) |
| Sex | |
| Male | 11 (50) |
| Female | 11 (50) |
| Ethnicity | |
| Malay | 17 (77.4) |
| Chinese | 3 (13.6) |
| Indian | 1 (4.5) |
| Iban | 1 (4.5) |
| Severity of AD | |
| Moderate | 9 (40.9) |
| Severe | 13 (59.1) |
Demographic Data of Patients, Including Age, Sex, Ethnicity, and Severity of Atopic Dermatitis
Likewise, the median dermal thickness remained similar across the assessment points (P = 0.597) with 0.72mm (range = 0.50 - 1.20 mm) at baseline, 0.77 mm (range = 0.53 - 1.10) at 2 weeks and 0.77 mm (range = 0.56 - 1.07) at 6 weeks of treatment (Table 3).
Itch and sleep loss also improved over the study period, with a significant positive correlation between itch severity and sleep loss (Rho = 0.491, P = 0.020). As the severity of the itch decreased, sleep loss also decreased.
4.2. Skin Atrophy
None of the patients exhibited skin wrinkling, increased skin transparency, increased shininess, telangiectasia, bruising, or loss of skin markings throughout the study (Table 3).
The median ultrasonographic thickness of the epidermis at baseline (0 weeks) was 0.40 mm (range = 0.30 - 0.50). After 2 weeks of continuous use of mometasone furoate 0.1% cream, the median epidermal thickness was 0.37 mm (range = 0.30 - 0.50). After 6 weeks of mometasone use, the median epidermal thickness was 0.40 mm (range = 0.30 - 0.50). There was no significant change in epidermal thickness throughout the 6-week use of mometasone (P = 0.311) (Table 4).
| Variables | 0 Week | 2 Weeks | 6 Weeks | P-Value |
|---|---|---|---|---|
| Epidermal thickness b, mm | 0.40 (0.30 - 0.50) | 0.37 (0.30 - 0.50) | 0.40 (0.30 - 0.50) | 0.311 |
| Dermal thickness b, mm | 0.72 (0.50 - 1.20) | 0.77 (0.53 - 1.10) | 0.77 (0.56 - 1.07) | 0.597 |
| Total skin thickness b, mm | 1.10 (0.83 - 1.70) | 1.10 (0.83 - 1.60) | 1.10 (0.87 - 1.57) | 0.638 |
| Skin erythema, % | < 0.001 | |||
| Score 0: Absent | 0 | 27.3 | 72.7 | |
| Score 1: Mild | 45.5 | 50 | 27.3 | |
| Score 2: Moderate | 45.5 | 22.7 | 0 | |
| Score 3: Severe | 9.1 | 0 | 0 | |
| Skin wrinkling, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | ||
| Skin transparency, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | 0 | |
| Telangiectasia, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | 0 | |
| Shininess, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | 0 | |
| Bruising, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | 0 | |
| Loss of skin markings, % | - | |||
| Score 0: Absent | 100 | 100 | 100 | |
| Score 1: Mild | 0 | 0 | 0 | |
| Score 2: Moderate | 0 | 0 | 0 | |
| Score 3: Severe | 0 | 0 | 0 |
Changes of Variables at Baseline (0 Weeks), 2 Weeks, and 6 Weeks after Treatment with Mometasone Furoate 0.1% Cream a
5. Discussion
In this study, all 22 subjects complied with the treatment protocol, and none experienced adverse events such as a burning or stinging sensation, swelling, contact dermatitis caused by mometasone furoate cream, delayed wound healing, or cutaneous infection at the application site. Compared to previous literature, our study did not reveal significant atrophogenic effects of mometasone furoate when assessed clinically and ultrasonographically.
There have been ongoing debates regarding the use of TCS and its potential for causing skin atrophy. Dykes et al. found no significant changes in skin thickness after 8 weeks of using fluticasone propionate 0.05% cream, also a potent topical corticosteroid, in 40 healthy adults, with only a 0.01-mm reduction (3%) compared to baseline (10). On the other hand, Korting et al. reported the skin-thinning effect of mometasone furoate 0.1% ointment with twice-daily application for 6 weeks without occlusion in 24 healthy adults, guided by 20MHz B-scan ultrasonography (11). Total skin thickness reduced by 12% on day 22 and 17% on day 29, compared to baseline (11). While previous literature has mostly focused on the relationship between TCS and their atrophogenic effects in healthy adults, our study added essential information regarding children with moderate-to-severe AD who require repeated use of TCS due to the chronic, relapsing nature of AD.
Topical corticosteroids exert inhibitory effects on fibroblasts, reducing the production of ground substance, type I, and type III collagen, thus decreasing dermal thickness (12). The severity of skin atrophy is determined by the potency of TCS, the frequency and duration of use, the anatomical area treated, and the vehicle used, such as ointment, cream, lotion, and gel (12). Mometasone furoate 0.1% cream is a potent steroid derived from hydrocortisone, designed to improve efficacy (11). Mometasone furoate exhibits greater anti-inflammatory activity and a longer duration of action compared to betamethasone (13). It is generally well-tolerated with only transient local adverse effects (13). Due to high lipophilicity and rapid hepatic biotransformation into 3 different metabolites with minimal intrinsic activity, it has low systemic availability via percutaneous absorption and has no significant effect on the hypothalamic-pituitary-adrenal axis (13). Moreover, mometasone furoate has a lower affinity toward dermal cells compared to epidermal cells, owing to its ester hydrolysis biotransformation in the skin (13). This contributes to its low corticosteroid receptor binding, resulting in minimal atrophogenicity (13). Chlorination at position-21 and esterification at position-17 enable mometasone to penetrate the stratum corneum to reach therapeutic concentrations in the skin without causing skin atrophy. Overall, mometasone furoate is an efficacious, potent corticosteroid with a low risk of local and systemic side effects (13). These features are hypothesized to contribute to its lower atrophogenicity.
In a systematic review by Li et al., steroid phobia among adult AD patients, parents, and caregivers of pediatric patients with AD ranged from 21.0% (95% CI, 15.8 - 26.2%) to as high as 83.7% (95% CI, 81.9 - 85.5%) (14). Lee et al. concluded that more patients with topical corticosteroid (TCS) phobia reported partial adherence to treatment (49.4%) and non-adherence (14.1%) (15). In comparison, patients without TCS phobia reported partial adherence in 29.3% of cases, while non-adherence was only 9.8% (15).
Five of the studies included in Li et al.'s research surveyed patients and caregivers about their concerns regarding TCS (14). The main concern reported regarding TCS was skin thinning (14). In our study, we did not observe any patients with evidence of skin thinning, both clinically and radiologically. The continuous use of mometasone furoate 0.1% cream for 6 weeks on the volar aspect of both forearms did not reveal any visible telangiectasia or increased skin transparency under dermoscopy. All 22 participants were directly observed for compliance with mometasone furoate cream application twice daily throughout the study period. Our study underscores the importance of vigilant clinical monitoring for signs of skin atrophy, as we did not observe any complications associated with the use of potent TCS in children during this study. Our clinical findings and dermoscopic examinations were further supported by ultrasonographic measurements of dermal and total skin thickness, which did not show any statistically significant reduction.
Lax et al. conducted a meta-analysis to compare the safety and effectiveness of different strategies for using TCS in the treatment of AD in children and adults (16). Sixty-three trials involving children with moderate-to-severe AD compared different potencies of TCS over a span of 1 to 5 weeks (16). Potent TCS resulted in a high treatment success rate of 70% compared to mild-potency TCS (16). Among the clinical trials that tested the treatment of eczema flare-ups, only 26 cases out of 2266 participants reported skin thinning (1%). These cases were associated with the use of higher-potency TCS: 16 with very potent, 6 with potent, 2 with moderate, and 2 with mild-potency steroids (16).
Smith et al. conducted cross-sectional surveys to assess the knowledge and attitudes regarding TCS use in pediatric AD among pharmacists (n = 292) and general practitioners (GPs) (n = 257) (17, 18). Only 27% of pharmacists and 46.9% of GPs would instruct parents to use TCS until remission (17, 18). Over half, 54% of pharmacists and 37.6% of GPs, advocated for sparing use of TCS (17, 18). Forty-six percent of pharmacists and 30.2% of GPs believed that skin atrophy was the most common side effect, and 56% of pharmacists also felt that side effects were unavoidable, even when TCS was used appropriately as directed by the treating physician (17, 18). Sixty-seven percent of pharmacists and 40.7% of GPs instructed parents not to exceed 2 weeks of continuous TCS use (17, 18). Contradictory messages from dermatologists, doctors, and allied health personnel may contribute to parental steroid phobia, treatment non-adherence, and poor treatment outcomes (17, 18). Our study demonstrated that potent TCS could be used continuously for more than 2 weeks in the pediatric population without clinical signs of skin atrophy. Evidence-based education among doctors and allied health personnel regarding potent TCS use in children is crucial for improving treatment compliance in this chronic, relapsing-remitting disease.
5.1. Strengths and Limitations of the Study
We conducted a prospective examination of the atrophogenicity of potent TCS, both clinically and radiographically, in children with moderate-to-severe AD over a 6-week duration. However, as a pilot study, we acknowledge some important limitations, including our small sample size. The major limitation of our study is that it was a prospective cohort study rather than a double-blinded, randomized controlled trial with a control group of healthy children without AD.
The use of the GE S7XD Clear L18Hz machine with a linear probe may have posed challenges in accurately measuring the epidermis and scale crust at the stratum corneum. This could possibly explain why we observed a good clinical response without a significant reduction in epidermal thickness in our patients.
Future studies should consider comparing different treatment durations, including periods beyond 6 weeks or even 12 weeks. Additionally, exploring different vehicles of topical mometasone furoate, other potent steroids, placebo, and topical calcineurin inhibitors may provide further insights into the atrophogenic effects of TCS in children with AD.
In conclusion, our study suggests that potent TCS like mometasone furoate 0.1% cream can be safely used in children with moderate-to-severe AD when used appropriately. Skin atrophy was not observed in our study, both clinically and radiographically, when topical non-occlusive mometasone furoate 0.1% cream was used continuously for 6 weeks. Regular clinical assessments by the treating physician, along with consistent messages on potent TCS use from all healthcare providers, are vital to alleviate parental steroid phobia and enhance treatment adherence.