1. Background
Palmoplantar keratodermas (PPKs) represent a group of skin disorders characterized by excessive epidermal thickening of palms and soles (1) and are classically divided into inherited or acquired forms. Inherited PPKs have familial occurrence and an early age of onset in the majority of cases. Acquired PPKs usually occur in adulthood, may be diffuse or focal, and are usually associated with inflammatory skin conditions, infections, internal malignancies, drugs, and other systemic diseases. Symptoms range from hyperhidrosis and malodorous maceration to fungal infections that can have a negative impact on the patient’s quality of life and lead to losing one’s job. Evaluation of PPK involves a clinical examination with medical and family history taking, histopathology assessments, and genetic testing. Family history may help identify hereditary PPK by disclosing an inheritance pattern. Dermoscopy is an office procedure used to magnify and visualize structures and patterns that are less visible to the naked eye, thus connecting clinical dermatology with microscopic dermatopathology (2).
Conditions causing PPK have a close morphological resemblance to each other and can be indistinguishable from each other clinically, a conundrum that can be resolved by dermoscopic examination and identifying distinctive diagnostic features. Dermoscopy has the potential to replace histopathology or may be more useful than histopathology in differentiating various causes of PPK (3).
2. Objectives
The main objective of this study was to investigate the clinical features and dermoscopic patterns of PPKs secondary to various dermatological conditions.
3. Methods
A cross-sectional study was performed on male and female patients in all age groups presenting with PPKs attending our dermatology outpatient department for 18 months from January 2021 to June 2022. A detailed history, including the age of onset, progression, involvement beyond palms and soles, association with other medical conditions, family history, systemic manifestations, and appendage involvement, was obtained from each patient. A clinical examination was carried out for all patients to gather relevant information, including morphological assessment of lesions, areas of involvement, associated dermatological diseases, and systemic symptoms, and confirm the diagnosis based on these findings. A biopsy was taken for histopathology examination if necessary. Dermoscopic assessment of the skin lesions was performed by a single observer using Heine Delta 20 Plus (10X) and FotoFinder Medicam 1000 (up to 140x). SPSS (Statistical Package for Social Sciences) version 20. [IBM SPSS statistics (IBM Corp. Armonk, NY, USA released 2011)] was used to perform statistical analyses. The chi-square test was applied to find any statistical association between qualitative variables. The level of significance was set at 5%.
4. Results
A total of 98 patients were evaluated, including 55 males (56.12%) and 43 females (43.87%), with a male-to-female ratio of 1.27:1. Office workers constituted the majority of the participants (62 patients, 63.26%). The age range of the patients was from 4 to 75 years, with a mean age of 39.5 years. The prevalence of various dermatoses causing PPK has been noted in Table 1, and their dermoscopic features have been summarized in Tables 2, 3, 4, and 5.
| Disease | No. (%) |
|---|---|
| Acquired palmoplantar keratoderma (n = 93) | |
| Palmoplantar psoriasis | 48 (50) |
| Palmoplantar dermatitis | 39 (40.62) |
| Pityriasis rubra pilaris | 3 (3.12) |
| Lichen planus | 2 (2.08) |
| Tinea manuum with pedis | 1 (1.04) |
| Total | 93 (100) |
| Hereditary palmoplantar keratoderma (n = 5) | |
| Ichthyosis vulgaris | 3 (3.12) |
| Greither’s syndrome | 1 (50) |
| Erythrokeratodermia variabilis | 1 (50) |
| Total | 5 (100) |
| Dermoscopic Findings | No. (%) | |
|---|---|---|
| Palms | Soles | |
| Background color | ||
| Light red | 16 (33.3) | 12 (25.0) |
| Dull red | 9 (18.8) | 11 (22.9) |
| Yellow | 2 (4.2) | 2 (4.2) |
| Light red + yellow | 21 (43.8) | 23 (47.9) |
| Scales | ||
| Diffuse white | 14 (29.2) | 19 (39.6) |
| Peripheral white | 2 (4.2) | 3 (6.3) |
| Patchy white | 10 (20.8) | 7 (14.6) |
| Diffuse yellow | 2 (4.2) | 2 (4.2) |
| Patchy yellow | 2 (4.2) | 1 (2.1) |
| Diffuse white + yellow | 5 (10.4) | 6 (12.5) |
| Peripheral white + yellow | 1 (2.1) | 0 (0.0) |
| Patchy white + yellow | 8 (16.7) | 8 (16.7) |
| Vascular pattern | ||
| Linear | ||
| Regular | 7 (14.6) | 4 (8.3) |
| Undifferentiated | 0 (0.0) | 2 (4.2) |
| Patchy | 0 (0.0) | 2 (4.2) |
| Dot | ||
| Regular | 8 (16.7) | 5 (10.4) |
| Irregular | 1 (2.1) | 3 (6.3) |
| Undifferentiated | 4 (8.3) | 3 (6.3) |
| Patchy | 0 (0.0) | 3 (6.3) |
| Glomerular | ||
| Undifferentiated | 8 (16.7) | 11 (22.9) |
| Patchy | 3 (6.3) | 4 (8.3) |
| Others | ||
| Yellow crusts | 2 (4.2) | 0 (0.0) |
| Hemorrhagic crust | 0 (0.0) | 1 (5.1) |
| Brownish orange globules | 0 (0.0) | 2 (4.2) |
| Dermoscopic Findings | No. (%) | |
|---|---|---|
| Palms | Soles | |
| Background color | ||
| Light red | 5 (12.8) | 7 (17.9) |
| Dull red | 5 (12.8) | 0 (0.0) |
| Yellow | 11 (28.2) | 9 (23.1) |
| Light red + yellow | 18 (46.2) | 22 (56.4) |
| Scale | ||
| Diffuse white | 4 (10.3) | 7 (17.9) |
| Peripheral white | 2 (5.1) | 0 (0.0) |
| Patchy white | 5 (12.8) | 2 (5.1) |
| Diffuse yellow | 1 (2.6) | 7 (17.9) |
| Patchy yellow | 3 (7.7) | 4 (10.3) |
| Diffuse white + yellow | 1 (2.6) | 5 (12.8) |
| Patchy white + yellow | 6 (15.4) | 13 (33.3) |
| Vascular pattern | ||
| Linear | ||
| Regular | 0 (0.0) | 2 (5.1) |
| Undifferentiated | 2 (5.1) | 0 (0.0) |
| Patchy | 1 (2.6) | 1 (2.6) |
| Dot | ||
| Regular | 1 (2.6) | 0 (0.0) |
| Irregular | 0 (0.0) | 1 (2.6) |
| Undifferentiated | 2 (5.1) | 6 (15.4) |
| Patchy | 3 (7.7) | 12 (30.8) |
| Glomerular | ||
| Regular | 4 (10.3) | 2 (5.1) |
| Undifferentiated | 0 (0.0) | 1 (2.6) |
| Patchy | 0 (0.0) | 5 (12.8) |
| Others | ||
| Orange dots | 2 (5.1) | 0 (0.0) |
| Yellow crusts | 0 (0.0) | 4 (10.3) |
| Hemorrhagic crust | 0 (0.0) | 2 (5.1) |
| Yellowish orange areas | 0 (0.0) | 1 (2.6) |
| Dermoscopic Parameters | Disease, No. (%) | ||
|---|---|---|---|
| Greither’s Syndrome (1) | Erythrokeratodermia Variabilis (1) | Ichthyosis Vulgaris (3) | |
| Background color | |||
| Light red + yellow | 1 (100) | 0 (0) | 0 (0) |
| Light red | 0 (0) | 1 (100) | 3 (100) |
| Scale | |||
| Diffuse white | 1 (100) | 1 (100) | 1 (33.3) |
| Patchy yellow | 0 (0) | 0 (0) | 1 (33.3) |
| Vascular pattern | |||
| Dotted irregular | 1 (100) | 0 (0) | 0 (0) |
| Linear regular | 0 (0) | 0 (0) | 1 (33.3) |
| Glomerular patchy | 0 (0) | 0 (0) | 1 (33.3) |
| Dermoscopic Parameters | Diagnosis, No. (%) | Total, No. (%) | P-Value | |
|---|---|---|---|---|
| Palmoplantar Dermatitis (n = 39) | Palmoplantar Psoriasis (n = 48) | |||
| Palmar dermatitis versus psoriasis, (palms) | ||||
| Background color | ||||
| Light red | 5 (12.80) | 16 (33.30) | 21 (24.1) | 0.026 |
| Yellow | 11 (28.20) | 2 (4.20) | 13 (14.9) | 0.002 |
| Scale | ||||
| Diffuse white | 4 (10.30) | 14 (29.20) | 18 (20.7) | 0.03 |
| Vascular | ||||
| Linear regular | 0 (0.00) | 7 (14.60) | 7 (8) | 0.013 |
| Dotted regular | 1 (2.60) | 8 (16.70) | 9 (10.3) | 0.032 |
| Plantar dermatitis versus psoriasis, (soles) | ||||
| Background color | ||||
| Dull red | 0 (0.00) | 11 (22.90) | 11 (12.6) | 0.001 |
| Scale | ||||
| Yellow | 9 (23.10) | 2 (4.20) | 11 (12.6) | 0.008 |
| Diffuse white | 7 (17.90) | 19 (39.60) | 26 (29.9) | 0.028 |
| Vascular | ||||
| Diffuse yellow | 7 (17.90) | 2 (4.20) | 9 (10.30) | 0.036 |
| Dotted regular | 0 (0.00) | 5 (10.40) | 5 (5.70) | 0.038 |
| Dotted patchy | 12 (30.80) | 3 (6.20) | 15 (17.2) | 0.003 |
Acquired PPKs constituted most of our cases (n = 93 out of 98), where the commonest etiology was palmoplantar psoriasis (n = 48, 50%), followed by palmoplantar dermatitis (n = 39, 40.62%), pityriasis rubra pilaris (n = 3, 3.12%), lichen planus (n = 2, 2.08%), and tinea manuum with pedis (n = 1, 1.04%). Hereditary PPKs were established in 5 patients, including ichthyosis vulgaris (n = 3, 3.12%), Greither’s syndrome (n = 1, 1.04%), and erythrokeratodermia variabilis (n = 1, 1.04%).
The commonest background color was light red and yellow (palm: 43.8%, sole: 47.9%) for psoriasis and (palm: 46.2%, sole: 56.4%) for eczema. The common scaling pattern in psoriasis was diffused white (palm: 29.2%, sole: 39.6%), whereas in eczema, it was patchy white with yellow (palm: 15.4%, sole: 33.3%). Vascular features in psoriasis showed regularly arranged dotted vessels as the commonest finding in the palms of 8 patients, whereas soles showed regular glomerular vessels in 11 patients. Eczema showed regular glomerular vessels as the commonest finding in the palms of 4 patients, whereas in soles, the most common finding was patchy dotted vessels. Pityriasis rubra pilaris revealed a light red background as the commonest finding (palm: 100%, sole: 66.7%). The scaling pattern showed diffused white scales (palm: 66.7%, sole: 66.7%). The vascular pattern of palms showed dotted vessels as the commonest finding (palm: 33.3%, sole: 33.3%). Lichen planus revealed light red with a yellow background in the palms of both patients, whereas in soles, a light red background was seen in both of them. Diffused white scales were common in the palms and soles of one of the patients. Regarding vascular features, irregular linear vessels were seen in palms and irregular dotted vessels in soles in 1 patient. Tinea manuum with pedis revealed a dull red background in both palms and soles. Diffused white-yellow scales were seen on the palms and soles, and patchy dotted vessels were seen in the soles of the patient. The dermoscopic patterns of hereditary PPKs have been described in Table 4.
5. Discussion
Clinically distinguishing different palmoplantar dermatoses is challenging, requiring histological examinations to provide a conclusive diagnosis (4). Dermoscopy findings in inflammatory dermatoses (inflammoscopy) have recently gained attention and can help reach a clinical diagnosis and reduce the need for biopsy (5, 6). This requires noticing important features when using dermoscopy for differentiating palmoplantar dermatoses, including scale patterns, morphology, arrangement of vascular structures, and any other specific feature (clues).
5.1. Palmoplantar Psoriasis
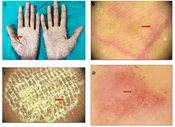
Psoriasis-associated PPK can manifest in a variety of morphological forms, from thick hyperkeratotic plaques to predominantly pustular lesions (7). The typical discoid lesions seen in hyperkeratotic PPK are regularly present on other parts of the body as well and are frequently a component of the general spectrum of psoriasis vulgaris (8). In our study, 48 patients (50%) had palmoplantar psoriasis (Figure 1). Dermoscopic examination revealed a light red and yellow background as the most common finding, followed by a light red background. This was somehow similar to the findings of Yu et al. (9) Also; we observed frequent whitish scales with a diffused pattern, which was similar to the observations of Lallas et al. (10) The vascular pattern revealed dotted and glomerular vessels, which were in line with other studies on dermoscopic features of psoriatic lesions (9, 11, 12). Red globular ring patterns were found in the palms of 11 patients and soles of 15 patients, which are considered highly specific for psoriasis (13).
A, erythematous scaly plaques of psoriasis distributed symmetrically over the palms, B, dermoscopy features of palmoplantar psoriasis, showing regular dotted vessels (the red arrow), C, dermoscopy features of palmoplantar psoriasis, showing characteristic diffused white scales (the red arrow) with a dull-red background, D, dermoscopy findings showing irregularly spaced rows of glomerular vessels (the red arrow) (Medicam1000, 20x).
5.2. Palmoplantar Eczema
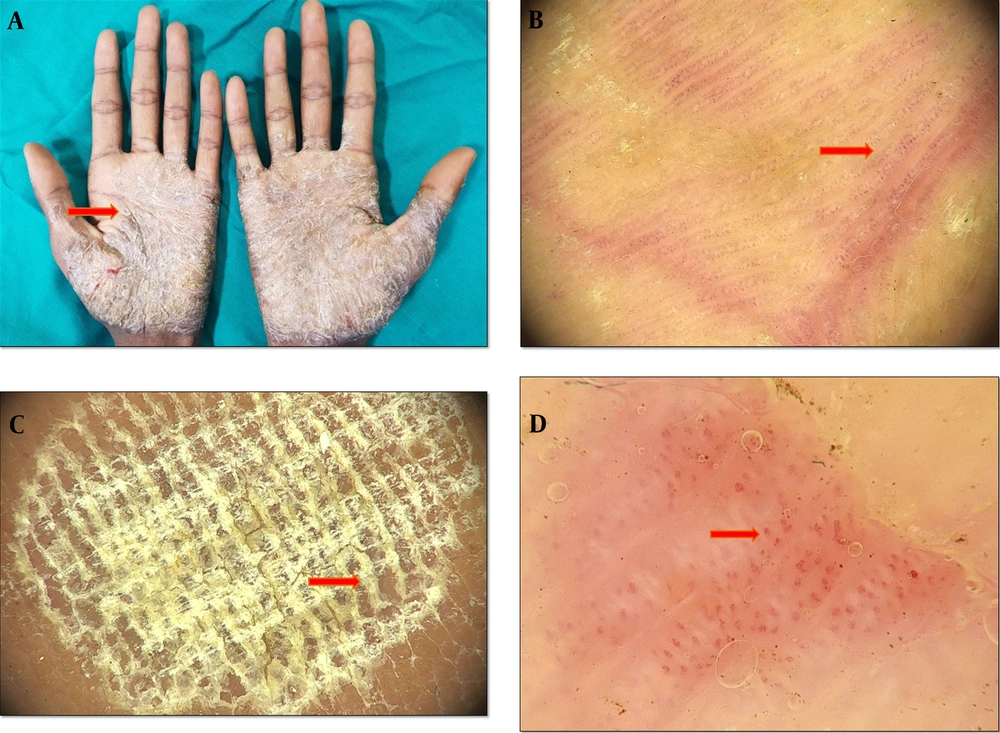
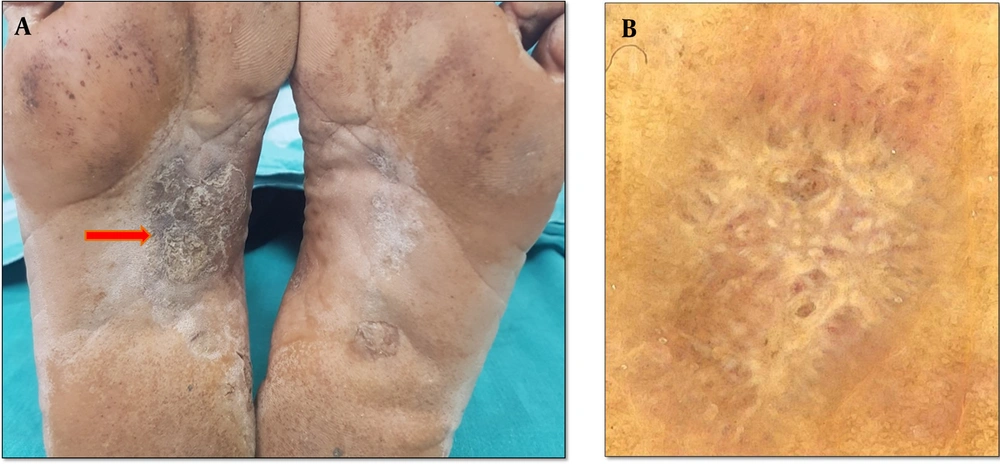
Hyperkeratotic eczema typically affects the palms, the volar parts of fingers, and occasionally, the plantar parts of the feet. Atopic dermatitis, allergic contact dermatitis, stasis dermatitis, and asteatotic eczema are a few examples of the various clinical entities that can be associated with eczematous dermatitis, all of which show the histological features of spongiosis (14-16). In our study, 39 patients (40.62 %) had palmoplantar eczema diagnosed based on clinical clues (Figure 2). Dermoscopic examination revealed a light-red and yellow background as the most common finding, followed by a yellow background. The light red and yellow background was seen in a higher ratio of our patients compared to a study by Cetinarslan et al. (17), who reported patchy white with yellow scales as the most common scale pattern, followed by patchy white and diffused yellow scales. These findings were in contrast to the reports of Cetinarslan et al. (17) and Errichetti and Stinco (11), who described that most scales were yellow in color. This could be due to ethnic-related skin color variations. The vascular pattern showed regular glomerular vessels, followed by patchy and undifferentiated dotted vessels. The higher incidence of dotted vessels with patchy distribution is on par with a previous study by Cetinarslan et al. (17).
A, hyperkeratotic scaly eczematous plaques with fissures distributed symmetrically over the plantar aspect of feet, B, dermoscopy findings of palmoplantar dermatitis showing patchy yellow (red) and white (purple) scales, C, dermoscopy examination showing dotted vessels (red) with a patchy distribution behind a light-red background, D, dermoscopy examination showing dotted vessels (the red arrow) in with clustered distribution and peripheral scales (the purple arrow) (Medicam1000, 20x).
5.3. Palmoplantar Psoriasis Versus Palmoplantar Dermatitis
Since most of our patients were diagnosed with either palmoplantar posoriasis or palmoplantar dermatitis, a comparison was made between the features of these two conditions, as summarized in Table 5.
To summarize, a light red/dull red background with diffused white scaling and regular linear, dotted, or glomerular vessels was in favor of palmoplantar psoriasis, while a yellow background, diffused yellow scaling, patchy dotted vessels, and yellow crust were more in favor of palmoplantar dermatitis. Almost similar findings have also been reported by Yu et al. (9).
5.4. Pityriasis Rubra Pilaris
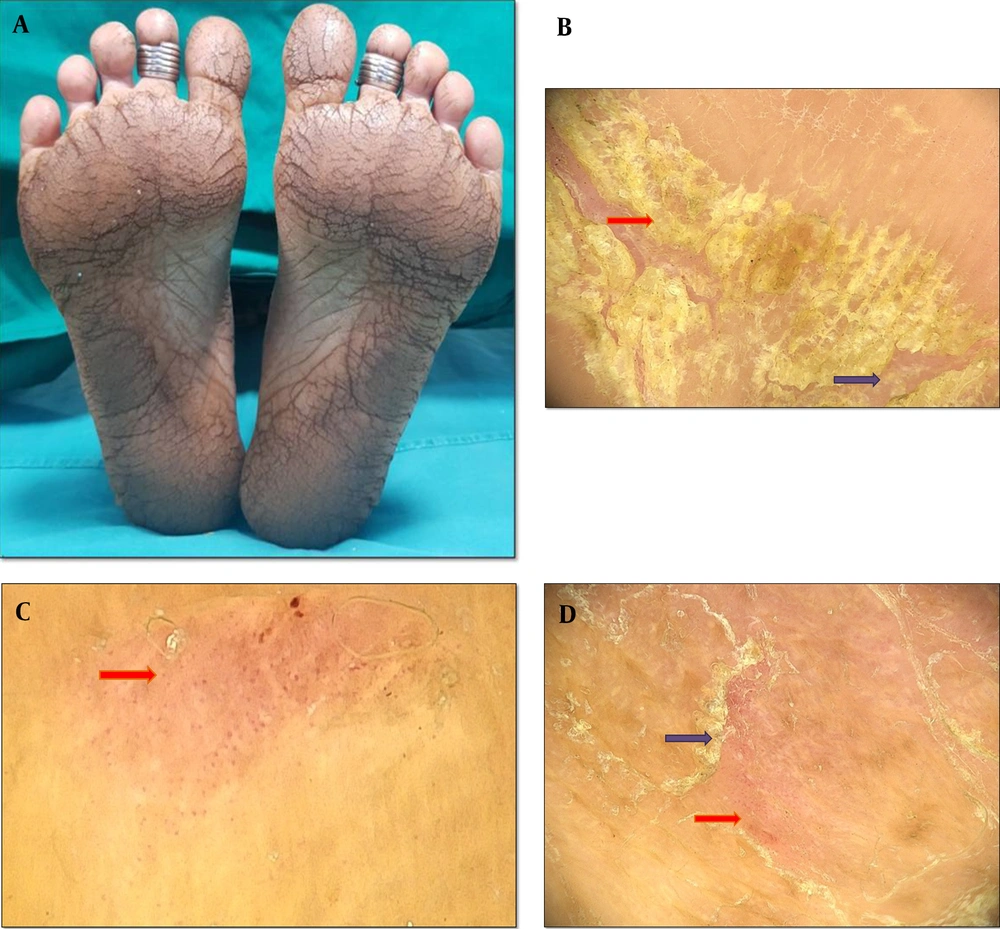
Red-orange waxy “sandal-like” PPK is characteristic of PRP. All three patients diagnosed with this condition had plantar involvement, and 2 of them had palmar involvement as well (Figure 3). A dermoscopic examination revealed a light red and orange background. The scale pattern showed diffused and patchy white scales. Errichetti and Stinco described the presence of unspecified whitish scales on dermoscopic examination of PRP lesions (18). The vascular pattern showed mixed morphology with regular linear vessels and patchy dotted vessels, as reported by Errichetti and Stinco. (18).
5.5. Lichen Planus
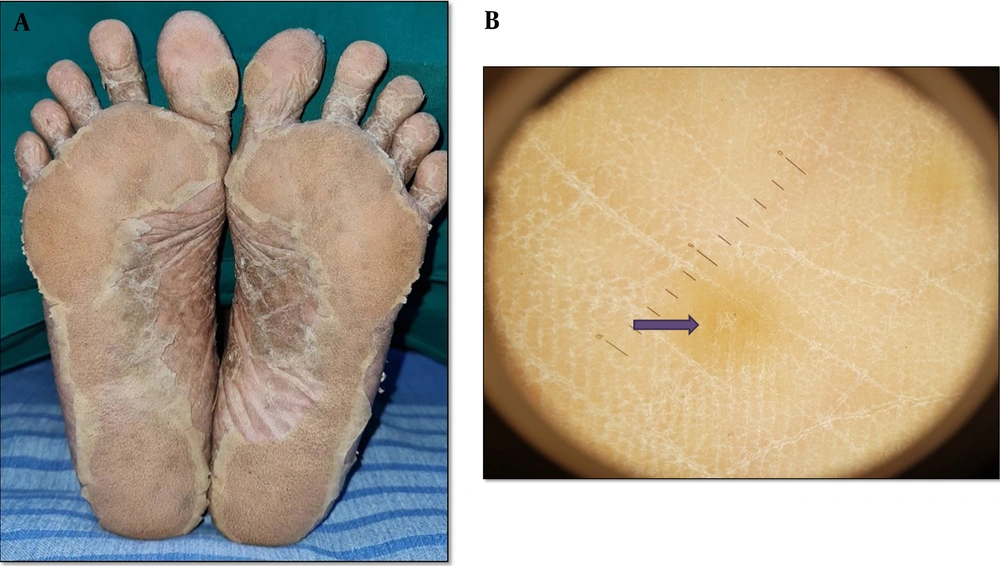
Palmoplantar LP displays a variety of clinical and morphological features, such as diffused scaly variant, hypertrophic variant, punctuate keratoses, diffused keratoderma, erosive or ulcerative lesions, pigmented macular variant, vesicular lesions, keratotic plaques with pits, and umbilicated papules (Figure 4A and B). Dermoscopic examination revealed a light red and yellow background in both patients. This was in accordance with a study by Makhecha et al., who reported a red background in early/active LP lesions (19). Diffused and peripheral white scales were also reported by Nayak et al. (20) Likewise, irregular linear (radial) and irregular dotted vessels were reported by Vazquez-Lopez et al. (21).
5.6. Tinea Manuum with Pedis
Scaling, erosion, and erythema of the inter-digital and sub-digital skin of feet with keratoderma of the instep are characteristic (22). In our study, one patient was diagnosed with PPK secondary to tinea manuum with pedis. Dermoscopic examination showed a dull red background with diffused white-yellow scales. The localization of the scales in skin furrows was characteristic, which was similar to a report by Errichetti and Stinco (22) The possible explanation might be the localization of dermatophytes, facilitating their proliferation in humid environments such as skin furrows. Patchy dotted vessels were also seen, which was similar to another study by Jakhar et al. (23).
5.7. Hereditary Palmoplantar Keratoderma
In our study, 5 patients were diagnosed with hereditary palmoplantar keratoderma (Table 1). Ichthyosis vulgaris is clinically characterized by extensive skin scaling with large, centrally adherent white scales with extensor distribution and is often accompanied by palmoplantar hyperkeratosis (24). All 3 patients with ichthyosis vulgaris revealed plantar involvement, and 2 of them had palmar involvement as well. A dermoscopy examination revealed a light red or dark background and diffused crisscross white and patchy yellow scales. The vascular pattern revealed regular linear and patchy glomerular vessels. Greither’s disease showed a light red and yellow background in dermoscopy examination, in addition to a diffused white scale pattern and multiple dotted vessels arranged in an irregular pattern. Erythrokeratodermia variabilis showed a background color of light red, diffused white scales with a crisscross pattern, and no distinctive vascular features. There is a paucity of studies on hereditary PPKs.
In our study, a skin biopsy for histopathological examination was performed when clinical and dermoscopic features were unable to confirm the diagnosis. So, a total of 13 biopsies were performed.
5.8. Limitations
(1) Most cases studied included psoriasis and eczema, but other conditions were relatively infrequent, so more studies are recommended on these rarer dermatoses.
(2) Only five of our patients were diagnosed with hereditary palmoplantar keratoderma during the study period, so it was not feasible to gather detailed dermoscopic findings of hereditary PPK.
(3) Histopathology was not performed in all cases, so we could not perform a correlational analysis between dermoscopic and histopathological findings.
5.9. Conclusions
This study sought to investigate the dermoscopic features of various causes of PPKs and evaluate if these patterns could distinguish between them. This is important as conditions such as psoriasis and eczema mimic each other very closely while they have different treatments. Although histopathology can aid in the correct diagnosis, overlapping histological features are not uncommon (4). Dermoscopy, a non-invasive procedure, can be useful in reaching the correct clinical diagnosis in this condition. The primary takeaway from this study is that dermoscopy can be a valid complementary tool for classifying various forms of keratodermas, particularly in situations where palmar/plantar involvement is the only or predominant manifestation of the disease. Furthermore, histopathology has a limited role in the diagnosis of PPK, whereas dermoscopy can be useful in establishing a clinical diagnosis. Whether dermoscopy can be a replacement for histopathology is a question yet to be answered in the future. Larger and more comprehensive studies on the patterns and special features of these conditions can also help answer this question.