1. Introduction
For decades, CO2 laser (10,600 nm) has served as a valuable surgical laser for incision, excision, vaporization, and coagulation of tissues by thermal mechanisms. However, some investigations have demonstrated that by some provisions, the CO2 laser can be used as a safe, non-destructive, non-invasive, and non-thermal laser for photobiomodulation therapy and immediate pain relief of some oral lesions with no thermal complications (1-10). This non-thermal, painless laser technique was termed Non-thermal CO2 Laser Therapy (NTCLT) afterward (8, 9, 11).
Over the past few decades, photobiomodulation (PBM), also known as photobiomodulation therapy (PBMT) or low-level laser therapy (LLLT), has been widely used as an effective, non-invasive, and safe therapeutic option in medical and dental practices. It is used to improve the healing process, provide pain relief, and reduce inflammation (12-14). In contrast to the high-power surgical lasers generally used in dermatological practice, the PBMT/LLLT systems are non-ablative and non-invasive, do not trigger temperature rise above 98°F, and do not induce tissue thermal damage (12).
Also, PBMT/LLLT has been described as the application of non-ionizing optical radiation with low-energy densities, absorbed by endogenous photoreceptors (such as cytochrome c oxidase in mitochondria) to provoke complex cascades of photochemical, photophysical, and photobiological events in cells leading to increased ATP production, modulation of reactive oxygen species, induction of transcription factors, and modulation of some biological reactions. These biological changes can provide favorable clinical therapeutic effects, such as pain relief, inflammation reduction, promotion of healing processes, and prevention of tissue death (12-14).
Currently, there is a large body of evidence supporting the valuable effects of PBMT/LLLT as a safe and non-invasive option for pain management of various painful conditions. Several systematic and narrative reviews have demonstrated the palliative effects of PBMT/LLLT on some oral lesions, such as recurrent aphthous stomatitis, burning mouth syndrome, and oral mucositis (15-18).
As known, PBMT/LLLT generally uses coherent light sources, such as He-Ne laser and semiconductor lasers, or non-coherent light sources consisting of filtered lamps or Light-emitting Diodes (LEDs), usually in the visible and near-infrared spectral range (12, 13). However, the considerable signs of progress in the field of PBM/LLLT have provided creative ways for the application of PBMT/LLLT. One of these signs of progress is the application of CO2 laser (10,600 nm) as a safe, non-destructive, and non-thermal PBM/LLLT device to provide significant and immediate pain relief of some kinds of painful oral and mucosal lesions such as recurrent oral aphthous ulcers, oral lesions of pemphigus vulgaris, and oral mucositis due to chemotherapy of solid tumors without any visible thermal adverse effects (1-9, 19). This non-thermal, painless laser technique was termed NTCLT afterward (8, 9, 11).
Consistent with the evidence of analgesic effects of NTCLT on some oral diseases, we decided to assess the analgesic effects of one session of NTCLT on leucopenia-induced oral ulcers of a kidney recipient patient with drug-induced leucopenia.
2. Case Presentation
We report a case of a 33-year-old female patient who received a kidney transplant from a deceased donor at the age of 30. She had an unremarkable post-transplant recovery period and was discharged with serum creatinine of 1.9 mg/dL. She was under maintenance immunosuppression with tacrolimus 3 - 4 mg daily to maintain its blood level within the narrow therapeutic range, Mycophenolate Mofetil (MMF) 1,500 mg daily, and prednisolone 2.5 mg per day. In the following two years, her kidney function improved, and her creatinine was between 1.3 and 1.6 mg/dL. Her Tacrolimus level was between 6.3 and 7.8 ng/mL. Two years later, she experienced some anemia, and her hemoglobin level decreased from 12 g/dL to 11 and later to 10.7 g/dL, and White Blood Cell (WBC) dropped to 3,400 per microliter. The routine workup showed some iron deficiency. Thus, iron was added to erythropoietin, and the dose of MMF decreased to 1 gr daily. She recovered fast, and blood elements returned to normal. Her WBC increased to 4,300, and the MMF dose was scheduled as before. However, three years after transplantation, she again experienced episodes of low WBC count, which did not respond well to MMF dose decrease, and even with discontinuation of it, her leucopenia continued. She was advised to be admitted. Finally, she was hospitalized in the transplant department due to fever, leukopenia, and painful oral ulcers.
Her labs showed a WBC count of 600 - 1000/mL, hemoglobin of 10.4 g/dL, and platelet count of 196,000/mL. She received antibiotics and a granulocyte colony-stimulating factor. Nine days after hospitalization, her WBC and hemoglobin increased to 6 900/mL and 10.9 g/dL, respectively. The creatinine level at discharge was 1.24 mg/dL. However, her aphthous lesions were very painful, and the extensive workups did not show any local infectious or systemic inflammatory accompanying diseases. Also, ANA, anti-ds-DNA, and anti-phospholipid antibodies were negative. However, her oral aphthous lesions did not improve; they were painful and interfered with her normal eating and drinking, and she could not brush her teeth at all.
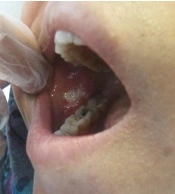
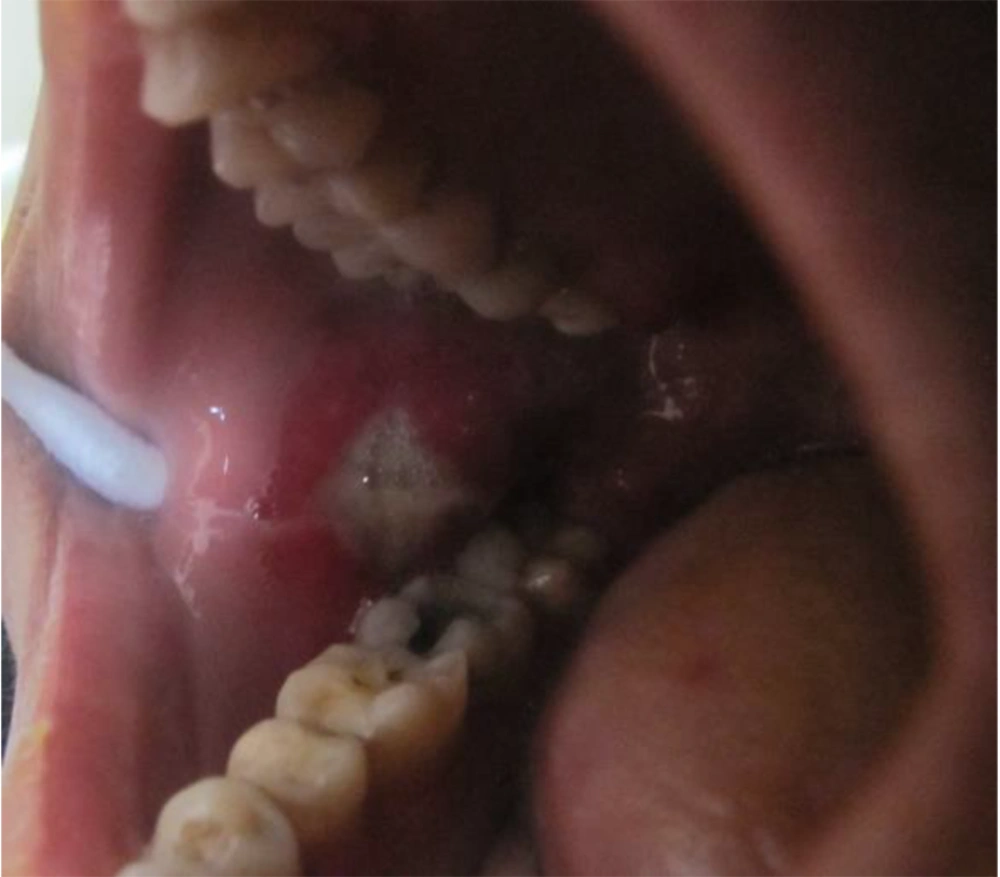
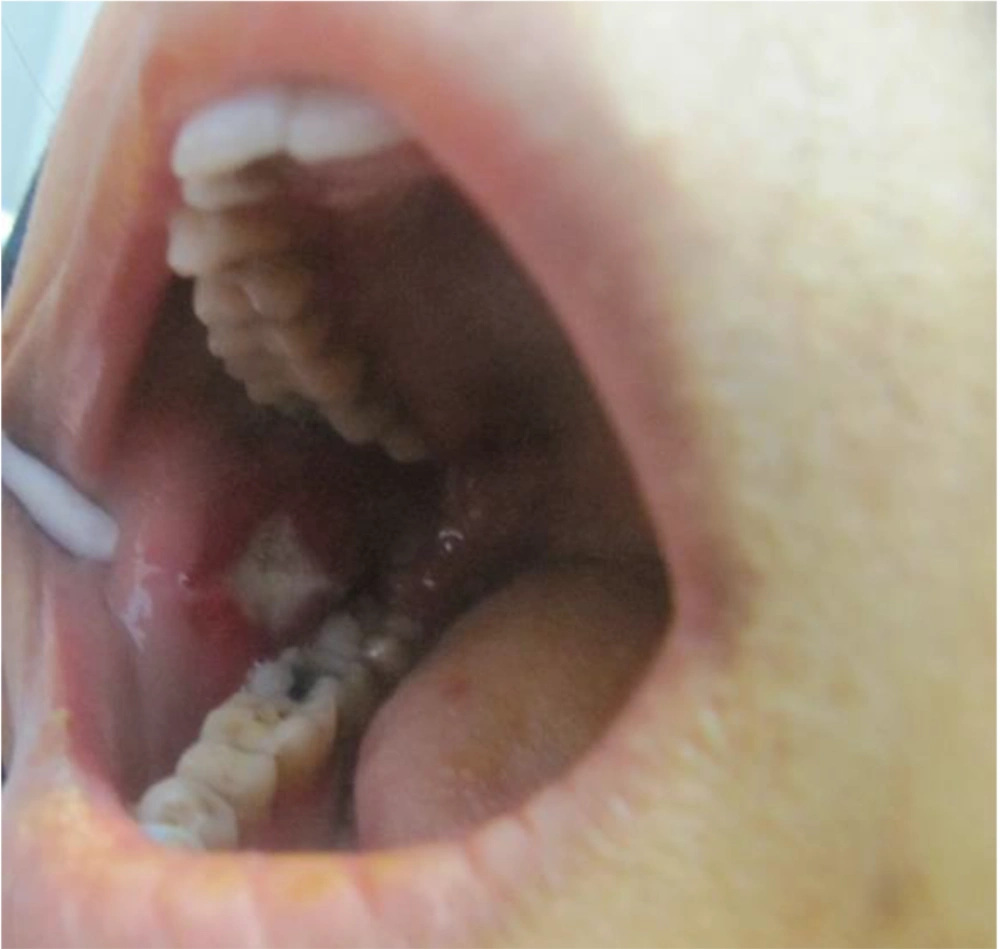
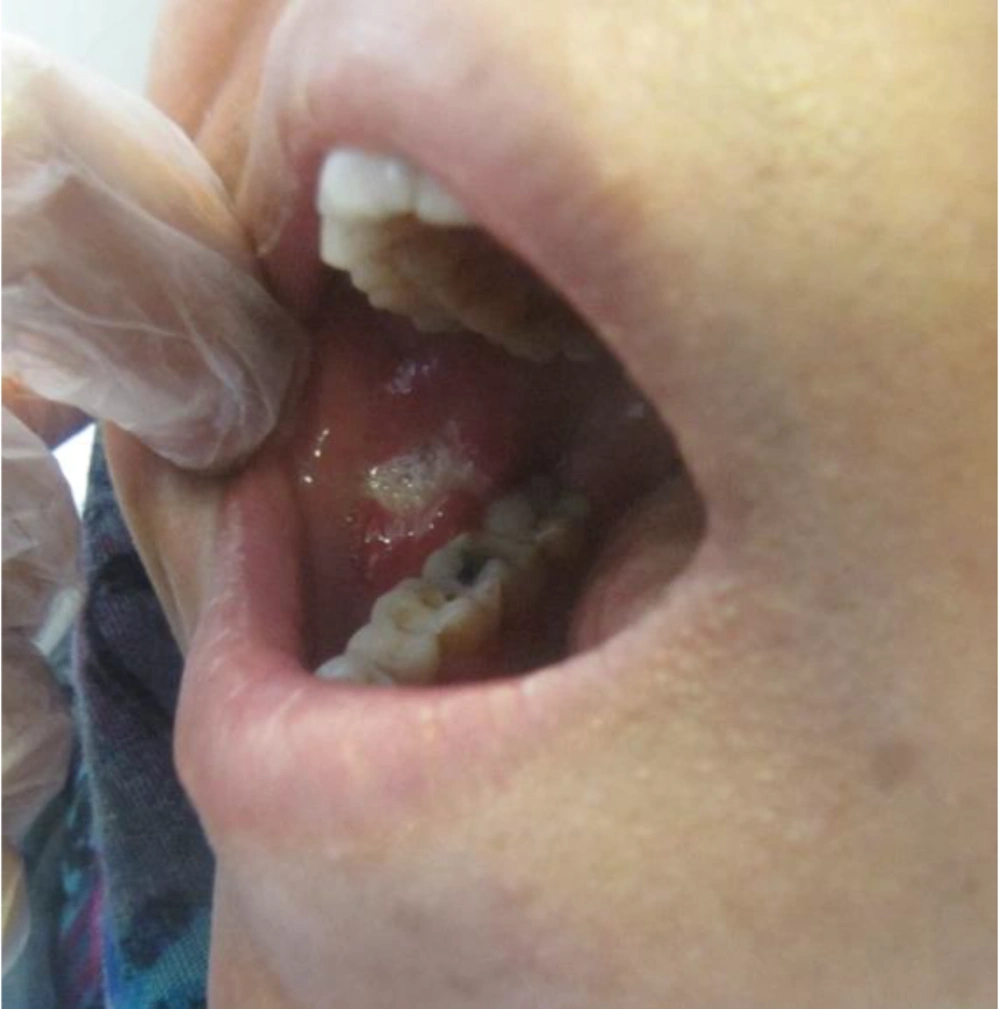
She was referred to our laser department for pain reduction of her oral ulcers by NTCLT. On clinical examination, there was a nearly rhomboid shape (not circular or oval) ulceration in her right cheek, with a white necrotic base and erythematous halo (Figure 1). The non-contact (non-stimulated) and contact pain scores of the ulcer were 6 and 8, respectively, on a horizontal, 10-cm visual analog scale (VAS). The non-contact (non-stimulated) pain Referred to the pain that the patient feels in the lesion without any mechanical or chemical stimulus. The term "contact pain" Referred to the pain experienced by the patient when the lesion is touched with a medical cotton swab. For pain relief of her right cheek ulcer, we used the NTCLT technique. Before CO2 laser irradiation, her right cheek ulcer and its surrounding tissue were covered with a thick layer (3 - 4 mm) of a transparent, colorless gel containing a very high percentage (87.5%) of water, which had no anesthetic effects. The patient and the medical staff put on protective safety goggles throughout the NTCLT application. The CO2 laser (λ = 10,600 nm; Lancet-2, Russia) was operated (power: 1 watt) with a defocused handpiece, scanning the surface of the lesion with a rapid circular motion of the handpiece. Immediately after NTCLT, the non-contact and contact VAS pain scores of the ulcer decreased significantly to zero and 1, respectively. No thermal side effects were observed after NTCLT (Figure 2). The procedure was completely painless, and neither systemic nor local anesthesia was required. During the next four days, the contact VAS pain scores were 2 - 3, and the non-stimulate pain score was zero with no adverse effects (Figure 3). The lesion showed complete healing within eight days.
There were two painful, irregular ulcers with a white necrotic base and minimal inflammatory halo on the lingual of mandibular incisors. Since they were not accessible for the CO2 laser handpiece, NTCLT could not be applied for them. The non-contact and contact pain of these two inaccessible ulcers were 8 and 10, respectively. These ulcers, which were considered as control, were irradiated with the same laser but with an inactive probe. Before NTCLT, the patient did not know which one of the lesions was going to be treated with NTCLT. No changes in the VAS pain scores were observed immediately after irradiation of the control lesions with inactive probe and until the next three days, and then gradually decreased over the next 7 - 9 days. These placebo lesions showed complete healing within 10 - 12 days.
Although it was planned that the patient would be blind to the treatment options for lesions (which one of the lesions was treated with NTCLT and which one was considered placebo and irradiated with inactive probe), it did not happen because of the rapid, dramatic, and instant pain relief that she experienced after treatment of the lesion with NTCLT.
3. Discussion
In this paper, we reported the immediate and significant analgesic effects of NTCLT on leucopenia-induced oral ulcers of a kidney recipient patient with drug-induced leucopenia. She was under maintenance immunosuppression with tacrolimus, mycophenolate mofetil, and prednisolone and was hospitalized due to fever, leukopenia, and painful oral ulcers. According to the clinical features of the oral lesions and the extensive workups, infectious diseases, and systemic inflammatory underlying diseases as the causes of oral ulcers were ruled out. Drug (tacrolimus/MMF)-induced leucopenia and the resultant leucopenia-induced oral ulceration were the most acceptable diagnoses for the patient.
Oral ulceration may be a major symptom in patients with leukopenia, and, in fact, it may be the first manifestation of drug-induced leukopenia. These lesions appear as painful, deep, irregular ulcers, often with a minimal inflammatory halo. Drug-induced oral ulcers can be a complication of immunosuppressive therapy in transplant patients. Oral ulcers have been recognized as an occasional complication of MMF and tacrolimus. There are at least 10 published case reports of MMF-induced oral ulcers in the literature (20).
The underlying mechanisms for tacrolimus, MMF-induced oral ulcers are not completely known yet. The most likely proposed mechanism is the direct cytotoxic and antiproliferative effects of tacrolimus/MMF on the oral mucosa. Another explanation is opportunistic infections by viral, bacterial, or fungal pathogens due to over-immunosuppression (20). Of note, in several cases, MMF-induced oral ulcers were accompanied by leukopenia (20, 21). The pain of these oral ulcers may be so severe that it interferes with eating, drinking, and speech. The resultant poor oral intake, weight loss, weakness, and even dehydration may be severe enough to warrant admission to the hospital for nutritional support, intravenous hydration, and pain management (20, 21). It seems rational to obtain novel analgesic options for these painful ulcers.
In this paper, we reported the immediate and significant pain-relieving effects of NTCLT on the painful oral ulcer of a kidney transplant patient with leukopenia. The procedure was completely pain-free, with no subsequent complications. In addition to this ulcer, the patient had two other painful oral ulcers, which were not accessible for the NTCLT procedure and were considered as control. The patient reported no pain relief after irradiation of these placebo lesions with an inactive probe, which confirmed the analgesic effects of NTCLT on the treated lesion.
PBMT/LLLT is increasingly recognized as an efficient and safe option for pain management of various painful conditions, including some kinds of oral lesions, such as recurrent aphthous stomatitis, burning mouth syndrome, and oral mucositis (15-18). Although semiconductor lasers (such as InGaAlP laser, GaAlAs laser, and GaAs laser) and LEDs are the most common PBMT/LLLT devices, some investigations found that by some provisions, surgical lasers can also be used for PBMT (22). As Tuner stated, "When high-power lasers are used for biomodulation, one only needs to make the beam wide enough not to burn. An alternative is to scan rapidly over the lesion with a narrow beam. Therefore, the power density or average power is kept low enough to avoid burning, and their incident energy and power density are set within the low-intensity laser therapy range" (22).
Also, CO2 laser, as a thermal, surgical laser, remains one of the most valuable lasers in surgery for cutting, ablation, and coagulation of tissues. Some studies have demonstrated that by some provisions, the CO2 laser can also be used as a non-thermal, non-invasive photobiomodulation laser for instant pain reduction of some oral and mucosal lesions without any visible complications (1-11). For application of CO2 laser as a PBMT/LLLT device, in addition to the valuable recommendations of Tuner (irradiation with a defocused handpiece or scanning of the lesion using a narrow beam), illumination of the laser beam through a completely transparent gel with a very high percentage of water can be another contraption for significant reduction of the final beam power by a factor of 200 - 500 (4, 19). By these provisions, the final beam power will be set within the PBMT/LLLT range under the gel layer and on the surface of the lesion (4, 19). This PBMT/LLLT technique was initially termed Non-ablative CO2 Laser Therapy (NACLT); however, after proving its non-thermal character, it was re-named Non-thermal CO2 Laser Therapy (NTCLT) (8, 9, 11) to avoid misconception with high-power, fractional non-ablative CO2 lasers used by dermatologists for cosmetic purposes such as skin rejuvenation and treatment of scars.
In the series of our previous studies, we reported the instant and significant pain-relieving effects of NTCLT in some oral and mucosal lesions such as minor and major recurrent aphthous stomatitis (RCTs) (3, 4), oral lesions of pemphigus vulgaris (before-after clinical trial) (8), oral and genital aphthous ulcers of Behcet's disease (case reports) (9, 11), and patchy chemotherapy-induced oral mucositis (in press). The results of these studies demonstrated the safe and painless nature of NTCLT. In this paper, we proposed another indication for NTCLT.
Although in this report, we used protective safety goggles matched to the CO2 laser wavelength (10,600 nm), we highly recommend the use of safety goggles that are matched to both the 10 600 nm wavelength and the guiding beam for comprehensive eye protection against the reflected beam from the gel surface.
3.1. Conclusions
In this paper, we reported the significant analgesic effects of NTCLT on leucopenia-induced oral ulcers of a kidney recipient patient with drug-induced leucopenia. The procedure was safe and pain-free, with no subsequent complications. This report can be valuable for dentists, dermatologists, nephrologists, and internists. Certainly, well-designed, high-quality, randomized controlled clinical trials (split-mouth design) will be able to confirm the analgesic effect of NTCLT on these painful oral ulcers.