1. Background
Skin candidiasis, also known as cutaneous candidiasis, is a common fungal infection that affects the skin. It is caused by the overgrowth of a yeast-like fungus called Candida, primarily Candida albicans (1). Candida is part of the normal flora in the human body, typically found in small amounts on the skin, mucous membranes, and gastrointestinal tract. However, under certain conditions, such as a weakened immune system or changes in the skin’s environment, Candida can multiply rapidly and cause an infection (2). Skin candidiasis can affect individuals of all ages; however, it is more commonly observed in warm and moist areas of the body, such as the armpits, groin, folds of skin, and areas under the breasts. It can also occur in areas where the skin is damaged, such as cuts, wounds, or areas affected by other skin conditions (3).
The symptoms of skin candidiasis can vary depending on the location and severity of the infection. Common signs and symptoms might include redness, itching, rash, and the formation of small pustules or blisters. In some cases, the affected skin might become cracked and scaly or develop satellite lesions around the main rash (4).
Risk factors for developing skin candidiasis include compromised immune system function, excessive sweating, obesity, diabetes, prolonged use of antibiotics or corticosteroids, and poor hygiene practices. Additionally, infants, elderly individuals, and those with certain underlying medical conditions are more susceptible to this type of fungal infection (5).
The diagnosis of skin candidiasis is typically based on clinical evaluation, where the healthcare provider examines the affected skin and might perform tests such as skin scrapings or cultures to confirm the presence of Candida (6). The treatment of skin candidiasis usually involves antifungal medications, such as topical creams or ointments, which are applied directly to the affected areas. In severe or recurrent cases, oral antifungal medications might be prescribed. It is also important to address any underlying factors contributing to the infection, such as managing diabetes or improving hygiene practices (7).
The prevention of skin candidiasis involves maintaining good personal hygiene, keeping the skin clean and dry, avoiding excessive moisture in skin folds, wearing breathable clothing, and practicing proper hand hygiene. As a result, skin candidiasis is a fungal infection caused by the overgrowth of Candida on the skin (8). The awareness of the risk factors, symptoms, and preventive measures can help in the early detection, treatment, and prevention of this common skin condition (9).
Candida albicans, the primary causative agent of skin candidiasis, employs complex pathogenic mechanisms. The study of skin candidiasis enables researchers to gain a better understanding of how Candida interacts with the host immune system, adheres to the skin surface, evades host defenses, and establishes infection (10). This knowledge can contribute to the development of targeted therapies and interventions for preventing or effectively treating the infection. The study of skin candidiasis is essential for various reasons, including understanding its impact on public health, unraveling the mechanisms of fungal pathogenesis, improving treatment approaches, addressing the needs of vulnerable populations, advancing diagnostic methods, and exploring innovative strategies to combat this common fungal infection (11).
Agglutinin-like sequence 3 (Als3) is a protein expressed by the opportunistic fungal pathogen C. albicans. Agglutinin-like sequence 3 belongs to a family of cell surface proteins known as the Als (agglutinin-like sequence) family, which play crucial roles in the adherence, colonization, and virulence of C. albicans (12). Agglutinin-like sequence 3 is one of the major adhesin proteins produced by C. albicans and is involved in the interaction of the fungus with host tissues. It plays a crucial role in mediating the adherence of C. albicans to various host cells and extracellular matrix proteins (13).
Agglutinin-like sequence 3 facilitates the binding of fungal cells to host epithelial and endothelial cells, thereby facilitating the establishment of infection. Moreover, Als3 has been shown to be a multifunctional protein with additional roles in the pathogenesis of C. albicans. It contributes to the formation of biofilms, which are complex communities of microorganisms embedded in a protective matrix (14). Biofilms enhance the resistance of C. albicans to antifungal agents and host immune responses, making them difficult to eradicate.
Furthermore, Als3 is known to stimulate host immune responses. It can interact with immune cells, such as dendritic cells, and trigger pro-inflammatory cytokine production, contributing to the initiation and modulation of host immune responses during Candida infections (15). Due to its critical role in adherence, colonization, virulence, and immune modulation, Als3 has emerged as a target for therapeutic interventions and vaccine development against C. albicans infections. Understanding the function and regulation of Als3 is crucial for developing new therapeutic approaches and preventive strategies against Candida-associated diseases (16).
Herbal compounds offer a natural and potentially safer alternative to conventional antifungal drugs. Many synthetic antifungal medications can have side effects or lead to the development of drug resistance. By exploring herbal compounds, researchers aim to identify effective and safe alternatives for the treatment of skin candidiasis (17). Herbal compounds often exhibit a broad spectrum of antimicrobial activity, targeting not only Candida species but also other fungi and microorganisms that might be involved in skin infections. This broader activity can enhance the efficacy of treatment and reduce the risk of recurrent or mixed infections (18).
Herbal compounds can serve as complementary or adjunctive therapies alongside conventional antifungal medications. Combining herbal compounds with conventional drugs might enhance overall antifungal activity, reduce the required dosage of synthetic drugs, and potentially minimize the risk of drug resistance. Herbal compounds might possess unique mechanisms of action that differ from conventional antifungal agents. Investigating these compounds can provide insights into new targets and pathways for the treatment of skin candidiasis. This knowledge can contribute to the development of innovative therapeutic strategies and the discovery of new drug candidates (19).
Molecular docking plays a crucial role in drug discovery and design, allowing researchers to optimize and prioritize compounds for further experimental validation, ultimately accelerating the development of novel therapeutics and providing a cost-effective approach to identifying potential leads (20). Investigating natural compounds against Als3 in the treatment of skin candidiasis is essential because it offers alternative therapeutic options to conventional antifungal agents. Candida albicans form biofilms using Als3, which enhances resistance to treatment. By targeting Als3, natural compounds can disrupt biofilm formation, increase susceptibility to treatment, and overcome antifungal resistance. Natural compounds have the potential for synergistic effects and a lower risk of adverse effects and can derived from readily available plant sources, making them accessible and potentially more sustainable. Overall, studying natural compounds against Als3 contributes to effective candidiasis management and expands treatment options.
2. Objectives
The purpose of this study was the in silico investigation of phenolic, terpenoid, saponin, alkaloid, polyphenolic, and naphthoquinone compounds as inhibitors against Als3 from C. albicans. Overall, the study likely aimed to provide insights into the potential of natural products as Als3 inhibitors, contributing to the understanding and development of alternative strategies for managing skin candidiasis.
3. Methods
3.1. Ligands Preparation
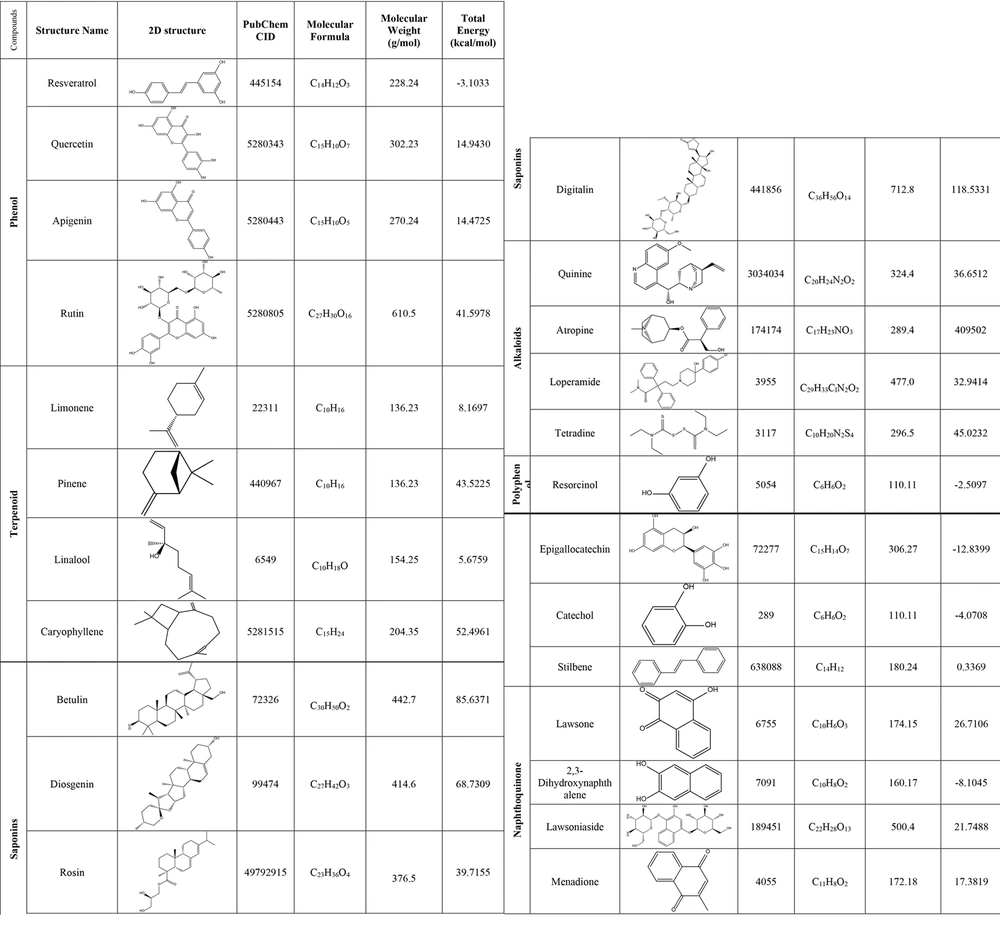
The two-dimensional (2D) structures of resveratrol, quercetin, apigenin, and rutin (phenol compounds), limonene, pinene, linalool, and caryophyllene (terpenoid compounds), betulin, diosgenin, rosin, and digitalin (saponin compounds), quinine, atropine, loperamide, and tetradine (alkaloid compounds), resorcinol, epigallocatechin, catechol, and stilbene (polyphenolic compounds), and lawsone, 2,3-dihydroxynaphthalene, lawsoniaside, and menadione (naphthoquinone compounds) as ligands were retrieved from the PubChem database in structure-data file (SDF) format (https://pubchem.ncbi.nlm.nih.gov/). The structures’ names, 2D structures, PubChem IDs, molecular formulas, and molecular weights of the compounds are reported in Figure 1. The 2D structures were converted from SDF to pdb format using ChemDraw Ultra 12.0.2.1076 software. The ligands were normalized with the mm2 method using Chem3D Pro 12.0.2.1076 software. After normalization, the total energy of all compounds was reported in Figure 1. Then, AutoDockTools 1.5.6 software was used to convert the initial format of the ligands from the .pdb to .pdbqt format (21).
3.2. Receptor Preparation
The 2D structure of Als3 adhesin from C. albicans (PDB ID: 4LEE) was obtained from the Protein Data Bank (https://www.rcsb.org/) with a resolution of 3.00 Å (Å: Angstrom) and in .pdb format. Subsequently, using Discovery Studio Client 4.5 software, the ligands and water molecules were separated from the main structure. Finally, the structure was prepared by adding polar hydrogens using AutoDockTools-1.5.6 software and saved in .pdbqt format (22).
3.3. Molecular Docking
The .config file was arranged, which was necessary for molecular docking. The size and location of the “grid box” were determined using AutoDockTools-1.5.6 software. Dimensions and centers (_X, _Y, and _Z) of the grid box were set to encompass all parts of the enzyme (4LEE: X center: 2.861, Y center: 1.139, Z center: 42.278). This was performed using the grid box tool in AutoDockTools-1.5.6 software. Then, the required information was recorded and saved in the .config file, and a specific .config file was created for each ligand (24 ligands). The overall results obtained through molecular docking were analyzed using AutoDockTools-1.5.6 software. To analyze the results further in detail, the ligand-receptor complex corresponding to the conformation with the highest binding energy was prepared and assessed for each compound using Discovery Studio Client 4.5 software (23).
4. Results
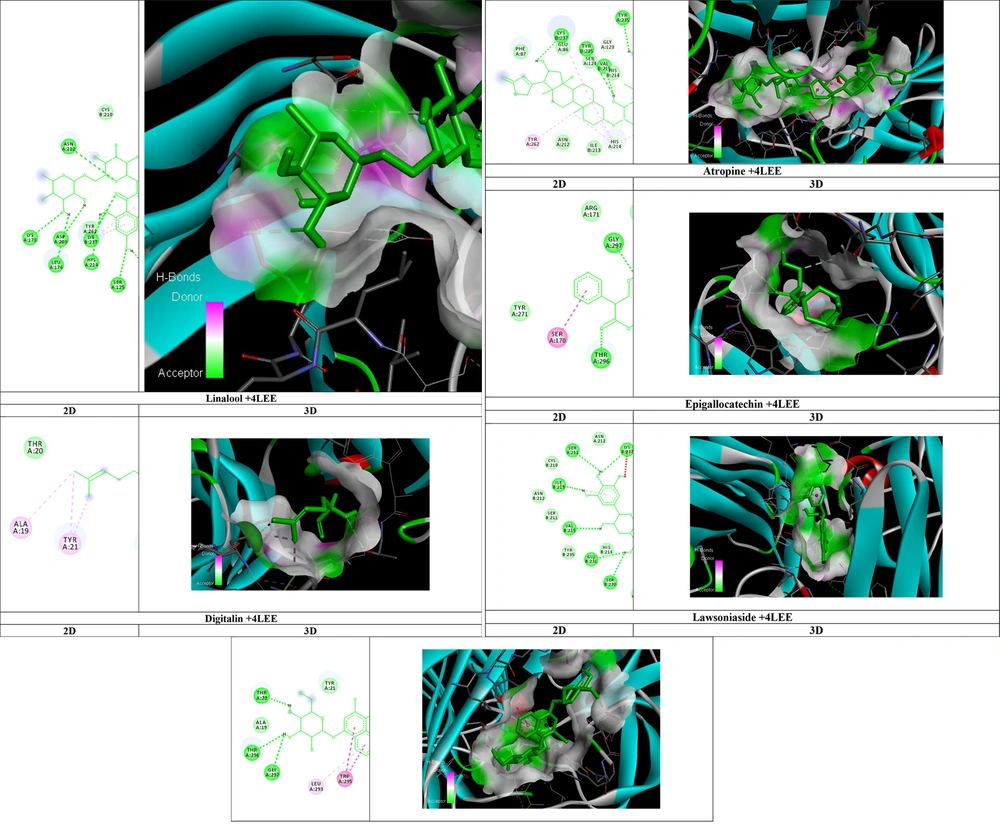
As shown in Table 1, the affinity of 4LEE with the distance from root mean square deviation (RMSD) and the best mode RMSD of the compounds were calculated, as depicted in Figure 2.
| Compounds | Affinity (kcal/mol) | D.F.R | B.M.R | H-bonds |
|---|---|---|---|---|
| Phenol | ||||
| Resveratrol | - 7.4 | 24.359 | 26.243 | SER:230/VAL:215/GLU:231; VAL:215/SER:230/SER:211; ASN:212 |
| Quercetin | - 9.1 | 2.203 | 3.325 | SER:230/GLU:231/TYR:235; SER:232/GLY:123/ILE:213; SER:211 |
| Apigenin | - 9.9 | 21.913 | 23.319 | SER:211/CYS:210/TYR:235 SER:232/VAL:215/SER:230 |
| Rutin | - 10.9 | 30.119 | 31.449 | ASN:212/LYS:178/ASP:209; LEU:176/LYS:237/HIS:214; SER:125/TYR:235 |
| Terpenoid | ||||
| Limonene | - 5.2 | - | - | - |
| Pinene | - 5.1 | - | - | - |
| Linalool | - 5.5 | 5.448 | 7.655 | THR:296 |
| Caryophyllene | - 4.6 | - | - | - |
| Saponin | ||||
| Betulin | - 7.7 | 1.952 | 7.756 | TYR:166 |
| Diosgenin | - 8.7 | 26.869 | 28.355 | TYR:255/SER:234 |
| Rosin | - 7.6 | 3.364 | 5.449 | TYR:226/ASP:169/TYR:23 |
| Digitalin | - 11.1 | 26.984 | 29.619 | LYS:237/TYR:235/VAL:215; TYR:235/SER:230/GLU:231; SER:230 |
| Alkaloid | ||||
| Quinine | - 7.8 | 2.273 | 3.810 | LYS:237/TYR:235 |
| Atropine | - 8.2 | 48.630 | 50.358 | GLY:297/THR:296 |
| Loperamide | - 5.2 | 48.606 | 51.704 | ASN:160 |
| Tetradine | - 4.1 | 49.372 | 51.041 | THR:20 |
| Polyphenol | ||||
| Resorcinol | - 7.9 | 27.704 | 27.998 | SER:230/VAL:215/SER:230; GLU:231 |
| Epigallocatechin | - 8.8 | 2.582 | 4.407 | SER:211/LYS:237/ILE:213; VAL:215/GLU:231/SER:230 |
| Catechol | - 6.9 | 23.351 | 23.505 | SER:230/VAL:215/GLU:231 |
| Stilbene | - 3.9 | - | - | - |
| Naphthoquinone | ||||
| Lawsone | - 8.4 | 2.781 | 4.485 | GLU:231/SER:230/TYR:235 |
| 2,3-Dihydroxynaphthalene | - 8.7 | 22.342 | 22.429 | VAL:215/SER:230/GLU:231 |
| Lawsoniaside | - 10.2 | 47.601 | 49.726 | TYR:23/ASP:169/TRP:224; THR:168/GLY:297/THR:296; THR:20 |
| Menadione | - 7.0 | 23.419 | 23.802 | SER:230/VAL:215 |
AutoDockVina Results of Phenolic, Terpenoid, Saponin, Alkaloid, Polyphenolic and Naphthoquinone Compounds
4.1. Phenol
Among these compounds, rutin with an affinity of -10.9 had the highest binding effect in the active site of 4LEE. Rutin combines with amino acids asparagine: 212H, lysine: 178H, aspartic acid:209H, leucine: 176H, lysine: 237H, histidine: 214H, serine: 125H, and tyrosine: 235H creating hydrogen bonds to inhibit the 4LEE protein.
4.2. Terpenoid
Among these compounds, linalool, with an affinity of -5.5, had the highest binding effect in the active site of 4LEE. Linalool combines with the amino acid threonine: 296H, creating hydrogen bonds to inhibit the 4LEE protein.
4.3. Saponin
Among these compounds, digitalin, with an affinity of -11.1, had the highest binding effect in the active site of 4LEE. Digitalin combines with amino acids lysine: 237H, tyrosine: 235H, valine: 215H, tyrosine: 235H, serine: 230H, glutamic acid: 231H, and serine: 230H creating hydrogen bonds to inhibit the 4LEE protein.
4.4. Alkaloid
Among these compounds, atropine with an affinity of -8.2 had the highest binding effect in the active site of 4LEE. Atropine combines with amino acids glycine: 297H and threonine: 296H, creating hydrogen bonds to inhibit the 4LEE protein.
4.5. Polyphenol
Among these compounds, epigallocatechin, with an affinity of -8.8, had the highest binding effect in the active site of 4LEE. Epigallocatechin combines with amino acids serine: 211H, lysine: 237H, isoleucine: 213H, valine: 215H, glutamic acid: 231H, and serine: 230H creating hydrogen bonds to inhibit the 4LEE protein.
4.6. Naphthoquinone
Among these compounds, lawsoniaside with an affinity of -10.2 had the highest binding effect in the active site of 4LEE. Lawsoniaside combines with amino acids tyrosine: 23H, aspartic acid: 169H, tryptophan: 224H, threonine: 168H, glycine: 297H, threonine: 296H, and threonine: 20H, creating hydrogen bonds to inhibit the 4LEE protein.
5. Discussion
Skin infections caused by C. albicans can significantly impact the quality of life, resulting in discomfort, pain, and psychological distress. Candida skin infections can manifest in various forms, including intertrigo, diaper rash, cutaneous candidiasis, and oral thrush, each with its distinct characteristics and affecting specific body areas. A comprehensive understanding of the clinical presentations and variations of Candida skin infections is essential for accurate diagnosis and proper management (24).
Although Candida skin infections are generally not life-threatening, they can lead to complications, especially in individuals with compromised immune systems or prolonged infections. If left untreated, the infection might spread to other parts of the body, potentially causing more severe systemic infections, such as invasive candidiasis. The timely diagnosis and treatment of Candida skin infections are crucial to prevent such complications (25).
Candida species, including C. albicans, have shown increasing resistance to commonly used antifungal agents. Antifungal resistance presents challenges in the treatment of Candida skin infections and underscores the need for alternative therapeutic approaches. Investigating the mechanisms of resistance and exploring new antifungal agents are critical steps in addressing the growing problem of antifungal resistance (26).
Agglutinin-like sequence 3 enables C. albicans to form biofilms, intricate communities of microorganisms embedded within a protective matrix. Biofilms enhance C. albicans’ resistance to antifungal treatments and host immune responses, making infections more challenging to eliminate (27).
Agglutinin-like sequence 3 interacts with host immune cells and modulates the immune response during Candida infections. It can stimulate immune cells, such as dendritic cells, triggering the production of pro-inflammatory cytokines. This immune modulation by Als3 has implications for the delicate balance between protective immune responses and tissue damage, influencing the outcome of Candida infections. Due to its role in adherence, colonization, and immune modulation, Als3 has been considered a potential target for vaccine development against C. albicans infections (28).
Furthermore, Als3 has been investigated as a diagnostic marker for Candida infections. The detection of Als3 or its specific antibodies in patient samples, such as blood or saliva, might indicate the presence of C. albicans infection. Agglutinin-like sequence 3-based diagnostic tests have the potential to enhance the accuracy and efficiency of Candida infection diagnosis, facilitating timely and appropriate treatment (29). In the meantime, the search for effective plant-based compounds can help alleviate the symptoms caused by skin infections. According to the results, the compounds rutin, linalool, digitalin, atropine, epigallocatechin, and lawsoniaside exhibited inhibitory effects against Als3. Among them, rutin was the most effective compound, forming the highest number of hydrogen bonds. A study conducted by Ivanov et al. evaluated the antifungal capacity of selected flavones (luteolin and apigenin), flavonols (quercetin), and their glycosylated derivatives (quercitrin, isoquercitrin, rutin, and apigetrin). Ivanov et al. reported that flavonoids have significant potential for further development as part of an anticandidal therapy or prevention strategy (30). This finding aligns with the results of the current study.
Furthermore, Herman A. and Herman AP. summarized the current state of knowledge on herbal products and their active constituents with antifungal activity against drug-resistant Candida spp. Herman A. and Herman AP. reported that herbal products and their active constituents have the potential to be effective against a wide variety of fungi, including drug-resistant Candida spp. (31). In this study, in addition to rutin, digitalin and lawsoniaside demonstrated similar effects as rutin. Digitalin inhibited Als3 and formed hydrogen bonds with amino acids lysine: 237H, tyrosine: 235H, valine: 215H, tyrosine: 235H, serine: 230H, glutamic acid: 231H, and serine: 230H. Yang et al. investigated the antifungal effects of saponin extract from the rhizomes of Dioscorea panthaica Prain et Burk (Huangshanyao Saponin extract, HSE) against C. albicans. Yang et al. reported that HSE might be used as a potential antifungal therapeutic against C. albicans (32).
Lawsoniaside inhibited Als3 and formed hydrogen bonds with amino acids tyrosine: 23H, aspartic acid: 169H, tryptophan: 224H, threonine: 168H, glycine: 297H, threonine: 296H, and threonine: 20H. In this regard, Janeczko et al. evaluated 1,4-Naphthoquinone derivatives that potently suppressed C. albicans growth and inhibited the formation of hyphae. The results of the aforementioned study showed that 1,4-Naphthoquinones significantly affected fungal strains at 8 - 250 mg/L of minimum inhibitory concentration (MIC) (33). These studies underscore the effects of herbal compounds, which are in line with the findings of this research.
The investigation of natural products as inhibitors of agglutinin-like sequence (Als3) from C. albicans holds significant implications for the treatment and management of skin candidiasis. If successful, these natural products could serve as potential therapeutic agents to combat the pathogenicity of C. albicans and prevent or alleviate the symptoms associated with skin candidiasis. Furthermore, the identification of specific natural products that exhibit inhibitory properties against Als3 could pave the way for the development of novel antifungal drugs derived from natural sources.
Despite the promising nature of this in silico study, several potential limitations should be considered. Firstly, in silico studies, studies rely on computational models and simulations, which might not fully capture the complexity and intricacies of the biological system. Therefore, further experimental validation is necessary to confirm the inhibitory effects of the identified natural products on Als3. Additionally, the study focused on the in silico characterization of Als3 inhibitors and did not address their pharmacokinetic properties, bioavailability, or potential toxicity. These factors need thorough evaluation before considering the clinical application of these natural products.
The findings of this in silico study pave the way for several future research directions. Firstly, the identified natural products should undergo rigorous experimental validation, including in vitro and in vivo studies, to assess their inhibitory activity against Als3 and their efficacy in treating skin candidiasis. Furthermore, researchers can investigate the structure-activity relationship of these natural products to optimize their inhibitory potency and selectivity.
Additionally, further studies can explore the potential synergistic effects of combining Als3 inhibitors with existing antifungal drugs to enhance therapeutic outcomes. Moreover, the study opens avenues for the discovery and investigation of additional natural products or synthetic compounds targeting other virulence factors of C. albicans. Finally, clinical trials and translational research are warranted to evaluate the safety and effectiveness of these Als3 inhibitors as a viable treatment option for skin candidiasis.
Rutin is a flavonoid with antioxidant and anti-inflammatory properties. It is generally considered safe for human use; however, availability and practicality for formulation might vary. Linalool is a terpene alcohol found in essential oils. It is generally regarded as safe but might cause skin irritation. Availability and practicality depend on specific applications and regulations. Digitalin is a potent cardiac glycoside derived from the foxglove plant. It requires medical supervision and is not readily available for self-use or formulation into over-the-counter products. Atropine, derived from Atropa belladonna, is used for medical purposes, such as pupil dilation and heart conditions. It should only be used under medical supervision and is not typically available for self-use or formulation. Epigallocatechin is a catechin found in green tea. It is generally safe when consumed in moderation as part of a balanced diet. Availability and practicality depend on specific applications and regulations. Lawsoniaside is a compound found in henna. Safety and practicality for medicinal or ointment use might require further research and evaluation.
5.1. Conclusions
This study has shed light on the potential of compounds such as rutin, linalool, digitalin, atropine, epigallocatechin, and lawsoniaside as strong inhibitors of Als3 in the treatment of skin infections through medicines, ointments, and washing liquids. The findings of this study provide an initial classification and description of the effects of herbal compounds against common skin lesions.