1. Background
Chronic wounds, which are disruptions in skin integrity that persist without resolution or require an extended period to heal, represent a significant health challenge (1). These wounds, including venous, arterial, diabetic, pressure, and traumatic ulcers, impose substantial costs in terms of personal, professional, and societal impacts. The financial expenditures and resource demands for the extended care of these chronic wounds constitute a global health concern, necessitating a comprehensive and scientifically rigorous approach to understanding and addressing their complex pathophysiology and varied causes. It is crucial to develop effective strategies to reduce the significant human, material, and economic burdens associated with these non-healing ulcers (2).
Autologous platelet-rich plasma (PRP) is a product derived from blood that is increasingly being used in clinical settings. Among its various applications, PRP has emerged as an alternative therapeutic option to traditional dressings for treating chronic ulcers. The therapeutic effectiveness of PRP is attributed to the high concentration of growth factors in platelets, which play an active role in tissue regeneration (3).
2. Objectives
The primary objective of this research is to thoroughly assess the efficacy of PRP in gel form for ulcer treatment. Additionally, an important part of this study is to evaluate the tolerance of patients to this PRP-based treatment method.
3. Methods
This prospective study, conducted from December 2021 to December 2023, includes cases of chronic ulcers in individuals aged 18 years and older. The study excludes cases showing signs of malignancy or infections.
The preparation of PRP and its conversion into a gel for ulcer treatment involve a systematic process using a centrifuge. The centrifugation follows the protocol established by Franco et al. (4):
- Initially, 8 mL of the patient's blood is drawn and divided equally between a blue tube (for PRP) and a red tube (containing thrombin).
- Both tubes are then subjected to an initial centrifugation at 340 × 10 bpm for 5 minutes, resulting in the separation of blood components and the collection of PRP in the blue tube.
- Next, the red tube, containing plasma and thrombin, is centrifuged a second time at the same speed and duration (340 × 10 bpm for 5 minutes).
- After centrifugation, 5 mL of PRP is drawn from the blue tube, which constitutes approximately 62.5% of the initial blood volume.
- This PRP is mixed with 2 mL of thrombin (25% of the initial blood volume) and 1 mL of 10% calcium gluconate (12.5% of the initial blood volume), and the careful mixing of these substances produces an activated PRP gel.
- The plasma is then heated to 37°C for 5 minutes to simulate physiological conditions and enhance the PRP's effectiveness.
- The resultant product, the activated gel, is thereafter ready to be applied to the ulcer wound.
The activated gel is subsequently ready for application to the ulcer wound. Healing outcomes were classified as follows: Complete healing (100% ulcer closure), very good healing (surface area reduction of 80 - 99%), good healing (surface area reduction of 60 - 80%), moderate healing (surface area reduction of 30 - 59%), poor healing (surface area reduction of 1 - 29%), and no response.
4. Results
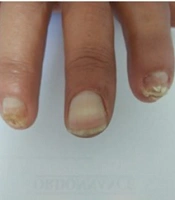
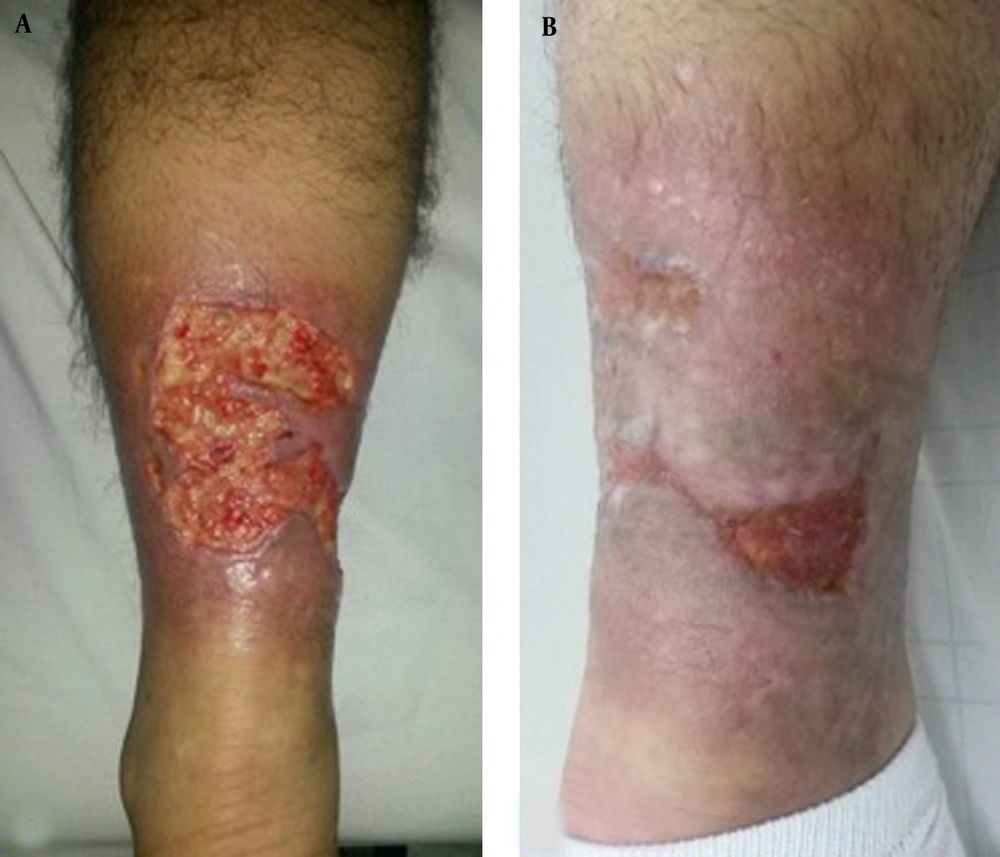
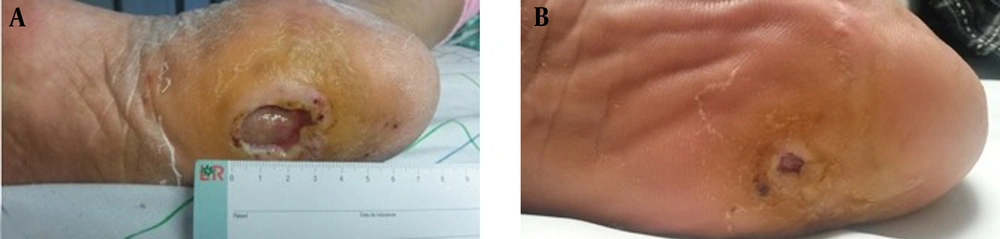
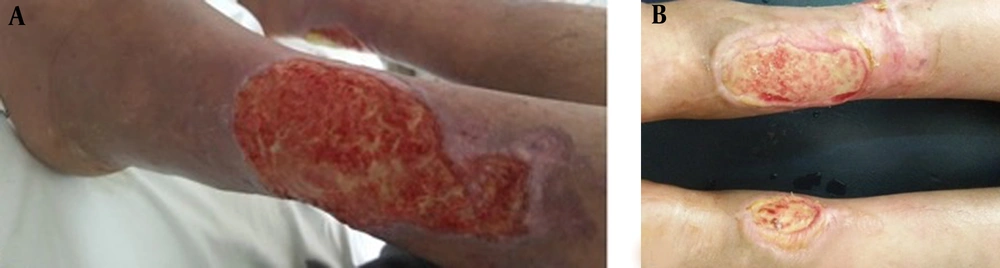
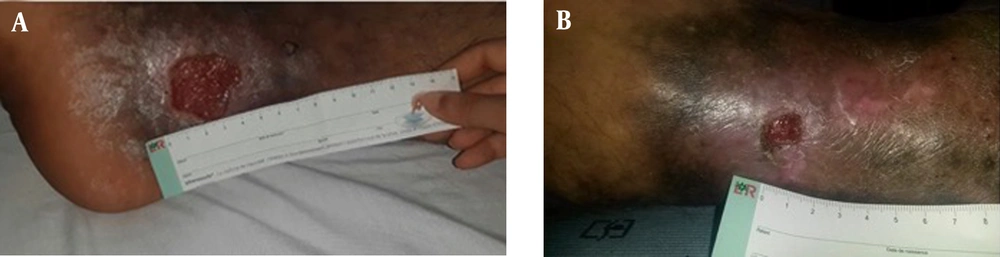
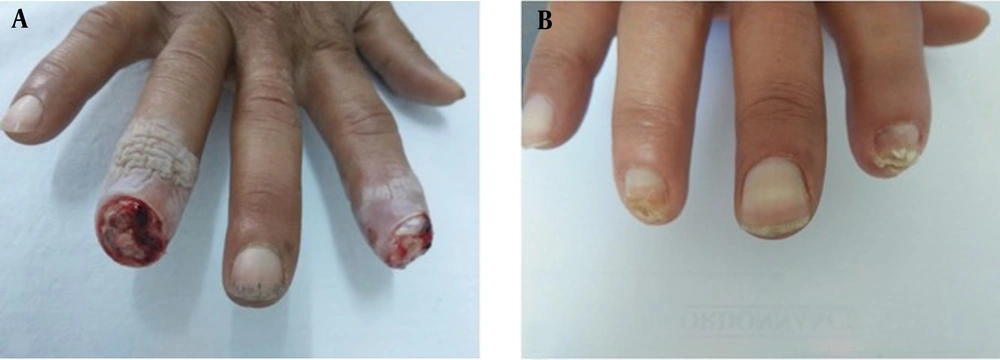
The study enrolled a total of 10 patients, consisting of 8 males and 2 females, with an average age of 48.8 years (ranging from 19 to 68 years). One patient, treated for severe Raynaud's phenomenon with a history of smoking and alcoholism, presented with digital ulcerations. Another patient had a history of chronic smoking, diabetes, COPD, and cardiomyopathy. The ulcers' etiology included one case of prolidase deficiency (Figure 1A), two cases of diabetic foot ulcers (Figure 2A), four cases of arterial ulcers (bilateral in one case: Figure 3A), two cases of venous ulcers (Figure 4A), and one case of Buerger's disease (Figure 5A). Diabetic patients achieved balance following endocrinologist consultation and insulin therapy.
The average duration of ulcer presence was 1 year and 2 months (ranging from 4 months to 3 years). The mean initial surface area of the ulcers was 18.7 cm2. Before applying PRP gel, two patients underwent necrectomy. On average, patients received 4 treatment sessions at 15-day intervals with Vaseline dressings, and the average healing time was 60.4 days. All patients maintained normal levels of serum albumin.
Complete healing was observed in one patient with Buerger's disease (Figure 5). Three patients experienced very good healing, five patients experienced moderate healing, and one patient with prolidase deficiency experienced poor healing (Figure 1). The introduction of dapsone after the second session led to moderate healing in the latter patient.
The average surface area after treatment for all patients was 8.05 cm2. No patients reported pain or adverse effects following the application of PRP gel. The average follow-up period was 5 months (Table 1).
| Patient | Gender | Age | Cause of Ulcer | No. of PRP Gel Applications | Initial Total Area (cm2) | Post-PRP Area (cm2) | Time of Healing (day) | Healing Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 (Figure 1) | M | 19 | Prolidase deficiency | 5 sessions + Disulone | 24 | 17.6 | 78 | Poor |
| 2 (Figure 2) | F | 46 | Diabetic foot ulcer | 4 sessions | 3 | 0.5 | 87 | Very good |
| 3 | M | 45 | Arterial ulcer (unilateral) | 4 sessions | 20 | 12 | 63 | Moderate |
| 4 | M | 58 | Diabetic foot ulcer | 3 sessions | 2 | 1 | 50 | Moderate |
| 5 (Figure 3) | M | 68 | Arterial ulcer (bilateral) | 5 sessions + necrosectomy | -40 (left leg); -10 (right leg) | -18; -5 | 65 | Moderate |
| 6 | M | 52 | Arterial ulcer | 5 sessions | 37 | 12 | 75 | Moderate |
| 7 | F | 57 | Arterial ulcer | 5 sessions | 24 | 8 | 79 | Moderate |
| 8 (Figure 4) | M | 34 | Venous ulcer | 1 session | 14 | 1.4 | 15 | Very good |
| 9 | M | 65 | Arterial ulcer | 4 sessions | 12 | 5 | 62 | Moderate |
| 10 (Figure 5) | M | 56 | Leo Buerger disease | 2 sessions + necrosectomy | 0.5 (index finger); 0.5 (ring finger) | 0 | 30 | Very good |
Abbreviation: PRP, platelet-rich plasma.
5. Discussion
The use of PRP in managing chronic ulcers is a relatively novel approach that has shown success in various medical fields, including orthopedics, dental and maxillofacial surgery, as well as in general and plastic surgery (5). It is important to note that PRP has few contraindications and is associated with minimal side effects (6).
Platelets contain a wide range of growth factors crucial for tissue repair. As a treatment option for chronic ulcers, PRP is recognized for its safety, ease of use, and cost-effectiveness, delivering promising outcomes (6). However, the lack of standardized protocols, along with challenges in product characterization and regulatory supervision, highlights the need for improvement and standardization in PRP production to enhance its effectiveness in treating chronic ulcers (Table 2).
| Study | Number of Patients | Method | No of Applications | Area (cm2) |
|---|---|---|---|---|
| Suthar et al. (2) | 24 | PRP gel | 1 subcutaneous injection and PRP gel topically for three days (24 weeks) | 0.5 - 10 |
| Cieslik-Bielecka et al. (7) | 5 | L-PRP | 1 after ten days | 200 - 300 |
| Moneib et al. (8) | 40 | PRP gel | 1 for three days (mean of 6 sessions) | < 10 |
| Escamilla Cardenosa et al. (9) | 58 | PRGF | Weekly for 24 weeks | 13.69 ± 30 |
Abbreviation: PRP, platelet-rich plasma.
The action mechanisms of PRP are as follows: Platelets are essential in clot formation during coagulation. After vascular injury, collagen from the adjacent connective tissue is exposed to the bloodstream. Along with other factors, this exposure initiates platelet aggregation and activation (10). When a clot forms, platelets release a range of growth factors, including platelet-derived growth factor (PDGF), insulin-like growth factor-1, epidermal growth factor, transforming growth factor-β, vascular endothelial growth factor, and fibroblast growth factors. Significantly, PRP has been shown to enhance the localized healing effects of these growth factors by supporting the growth of granulation tissue, stimulating mechanisms for collagen production, encouraging the gathering of fibroblasts, macrophages, and other cells, and aiding in the formation of new epithelium (11).
The study by Sakaria and Alva (12) explores the therapeutic effectiveness of PRP in treating chronic wounds and ulcers. The study involved 25 patients who received a single PRP injection alongside the topical application of autologous platelet gel. Significant outcomes included a notable decrease in wound size for all participants, with a remarkable 56% of them showing a reduction of more than 90% in wound dimensions within just four weeks of starting treatment. Importantly, there were no adverse complications linked to the PRP treatment, supporting previous research that identifies PRP as a safe and effective approach for treating non-healing wounds and ulcers.
Furthermore, a systematic review and meta-analysis conducted by Xiong et al. (13) indicated that PRP application leads to a significant reduction in ulcer size and improved wound healing rates in patients with venous leg ulcers specifically. This finding aligns with consistent outcomes across studies, highlighting the therapeutic advantages of PRP in managing chronic wounds, especially venous leg ulcers.
Saad Setta et al. (14) performed a comparative study of PRP and platelet-poor plasma (PPP) on individuals dealing with chronic diabetic foot ulcers. Their research revealed significantly faster healing rates associated with PRP treatment, suggesting that PRP may enhance cell migration and proliferation, contributing to the rapid healing of chronic ulcers. Notably, in our study, PRP was applied every two weeks, contrasting with the more common practice found in the literature of applying it twice weekly (15, 16). This divergence in application frequency underscores the varied approaches to PRP treatment in the clinical setting.
In our study, the application of PRP acted as a significant facilitator in the healing of chronic ulcers. However, it's crucial to acknowledge that assessing its impact on specific types of ulcers was limited by the small sample size. The effectiveness of this approach relies on the localized and prolonged release of various PDGFs and proteins, reflecting the complexity of the natural healing process (17). The use of PRP seems to counteract the adverse effects of deep tissue injury and serves as a preventative measure against further tissue necrosis. These results highlight PRP's potential as a therapeutic option for chronic ulcers, suggesting the need for further research with larger patient groups to clarify its effectiveness across different ulcer categories.
The literature indicates that PRP might positively affect the healing of diabetic foot ulcers (18). However, it's important to note that this evidence comes from small-scale randomized controlled trials of relatively lower quality (1). Despite the small size of our study, we noted significant progress in treating four patients with diabetes, indicating a promising and favorable outcome.
Contrary to expected findings, Ramos-Torrecillas et al. (19) found no clear link between blood albumin or total protein levels and the healing effects of PRP. This observation is in line with the results reported by de Leon et al. (20), which suggest that serum albumin or hemoglobin levels do not significantly influence the effectiveness of plasma growth factors on chronic ulcers. Notably, none of the patients in our series showed signs of hypoalbuminemia. These insights add valuable context to the ongoing discussion about PRP's role and the various patient factors involved, emphasizing the importance of a detailed understanding of its therapeutic capabilities.
Our series presents unique characteristics that illustrate the wide-ranging applications of PRP in treating ulcers. For instance, PRP showed promise in treating leg ulcers caused by prolidase deficiency in one patient, offering a new insight into its effectiveness for particular medical conditions. Moreover, the use of PRP for a patient with digital ulcers due to severe Raynaud's phenomenon emphasizes its potential in addressing specific clinical challenges.
Highlighting the need for etiological treatments for ulcers, our series stresses the importance of tackling the root cause in addition to symptomatic treatment. Investigating PRP's role in alleviating ulcer-related pain expands its benefits beyond just wound healing.
Additionally, the series points out the variability in the quality of PRP, emphasizing the impact of preparation techniques and the patient's health background, especially in diabetic patients. This variability highlights the critical need for precise PRP preparation protocols and careful consideration of underlying health conditions in its application.
Although our study provides valuable insights into the efficacy of PRP therapy for chronic ulcers, it's crucial to recognize certain limitations for a comprehensive understanding of the results. Despite the study's strengths, such as assessing PRP's efficacy in conditions beyond Buerger's disease and prolidase deficiency, the relatively small sample size and the lack of a control group necessitate further research. These limitations highlight the need for more studies to clarify PRP's role in ulcer treatment and to refine treatment protocols for better outcomes across various patient groups.
In conclusion, our series emphasizes the necessity of further detailed studies to deepen our understanding of PRP's specific uses, investigate its effects on pain relief, and thoroughly evaluate its efficacy in distinct medical scenarios.