1. Background
Burns are painful skin injuries caused by thermal, chemical, and electromagnetic contact (1). Burn wounds represent a significant global health issue, resulting in thousands of deaths worldwide each year (2). These injuries not only impact individuals physically but also psychologically, affecting their quality of life (3). Burns are classified into four categories based on the depth of skin involvement: First-degree burns, affecting only the epidermis, also known as superficial burns. Second-degree (partial-thickness) burns involve the epidermis and a portion of the dermis. Second-degree burns can further be categorized as superficial (involving the epidermis and superficial dermis) and deep second-degree burns (involving deeper layers of the dermis, hair follicles, and glandular tissue). Third-degree burns entail destruction of the epidermis and dermis layers, potentially involving subcutaneous tissue, also referred to as full-thickness burns. Fourth-degree burns extend beyond the epidermis, dermis, and subcutaneous tissue to affect underlying elements such as bones, muscles, and tendons (4, 5).
The process of burn wound healing is complex and involves tissue repair and reconstruction. This process occurs in three overlapping phases: Inflammation, proliferation, and remodeling. The duration of burn wound healing varies depending on the degree of the burn (1). Second-degree burns typically take 1 to 3 weeks to heal and are the most common type of burns. Potential complications associated with second-degree burns include infection and the development of abnormal scarring (6). Despite significant advancements in burn wound treatment, complications remain common, underscoring the importance of discovering and developing safe and effective therapeutic agents for burn treatment.
Cinnamic acid (CA) is an organic acid found in many plants and fruits. Several studies have reported its antioxidant, antimicrobial, anti-inflammatory, antidiabetic, anticancer, and wound healing properties (7-9). In a previous study, we investigated the effect of CA on full-thickness wounds in rabbits, revealing that CA accelerated wound healing (8).
2. Objectives
The main objective of the present study was to compare the effects of CA and silver sulfadiazine (SSD) on deep second-degree burns in rabbits.
3. Methods
3.1. Preparation of CA Ointment
Cinnamic acid (Catalog Number: 800235) was purchased from Merck Millipore Co. (Germany). Cinnamic acid ointments at concentrations of 0.1%, 0.5%, and 1% (w/w) were prepared using the levigation method. Briefly, the CA powder was triturated with a mortar and pestle along with a small amount of glycerin. After triturating the CA powder, eucerin, the ointment base, was placed on one side, and CA powder on the other side. A small amount of powder was mixed with a portion of eucerin, and they were thoroughly and uniformly blended until a homogeneous mixture was obtained.
3.2. Animals
Fifteen female and male adult New Zealand white rabbits weighing 2 to 2.3 kg were purchased from the animal center of Hamadan University of Medical Sciences (Hamadan, Iran) for use in this study. The animals were individually housed in cages for one week prior to the start of the study. They were kept under controlled environmental conditions (temperature 25 ± 2°C, relative humidity 60 ± 5%, and a 12:12 hour light-dark cycle). The rabbits had free access to water and standard food. The ethics committee of Hamadan University of Medical Sciences approved the experimental procedures for this study (IR.UMSHA.REC.1398.1028).
3.3. Burn Wound Induction
To induce a deep second-degree burn, the wound site was first determined in the flank area of the rabbit's body. The hair in the wound area was completely shaved, and then the specified area was disinfected with 70% ethanol and anesthetized with a subcutaneous injection of Lidocaine 2%. A circular metal plate with a diameter of 1.2 cm and a thickness of 5 mm, preheated to 100°C, was maintained in contact with the rabbit's skin for 20 seconds to induce a second-degree burn (10, 11). Two wounds were created on the back of each animal. The animals were randomly divided into 5 equal groups, with each group consisting of 3 animals and 6 wounds. The first group was treated with SSD as the standard control, while the second, third, and fourth groups were treated with ointments containing 0.1%, 0.5%, and 1% CA in a eucerin base, respectively. The fifth group was treated with eucerin alone. Treatments were administered topically twice a day starting 3 hours after burn induction and continued for 24 days.
3.4. Wound Contraction Rate Assessment
To evaluate wound contraction rate, photographs were taken of the burn wounds on days 1, 3, 6, 10, 14, 17, 21, and 24 post-wounding. The wound area was calculated using the image processing program ImagJR. Wound contraction percentage was calculated by subtracting the area of the wound on the specific day from the area of the wound on the first day and multiplying by 100.
3.5. Determination of Collagen Content in Tissue Samples
Tissue specimens were collected from the experimental groups on days 7 and 24 after wounding. The Hydroxyproline assay kit (KiaZist Life Sciences, Iran) was used to measure the amount of collagen in tissue samples. Hydroxyproline, found exclusively in collagen protein, serves as an indicator of collagen content in the sample. Measurement of hydroxyproline was conducted according to the manufacturer's instructions.
3.6. Histopathological Study of Tissue Samples
Samples were collected from each experimental group on days 7 and 24 after burn wound creation. The tissue samples were fixed in 10% formalin, Paraffin blocks were made, and sections of 5 µm were obtained and stained with Hematoxylin and Eosin (H&E) dyes. A quantitative assessment of the histological state of the healing burn, including inflammation, angiogenesis, granulation tissue formation, and epithelialization, was conducted on tissue samples.
3.7. Statistical Analysis
Data analysis was performed using one-way and two-way analysis of variance (ANOVA) followed by multiple comparison tests. GraphPad Prism software was used for data analysis.
4. Results
4.1. Wound Contraction Rate
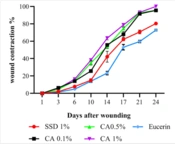
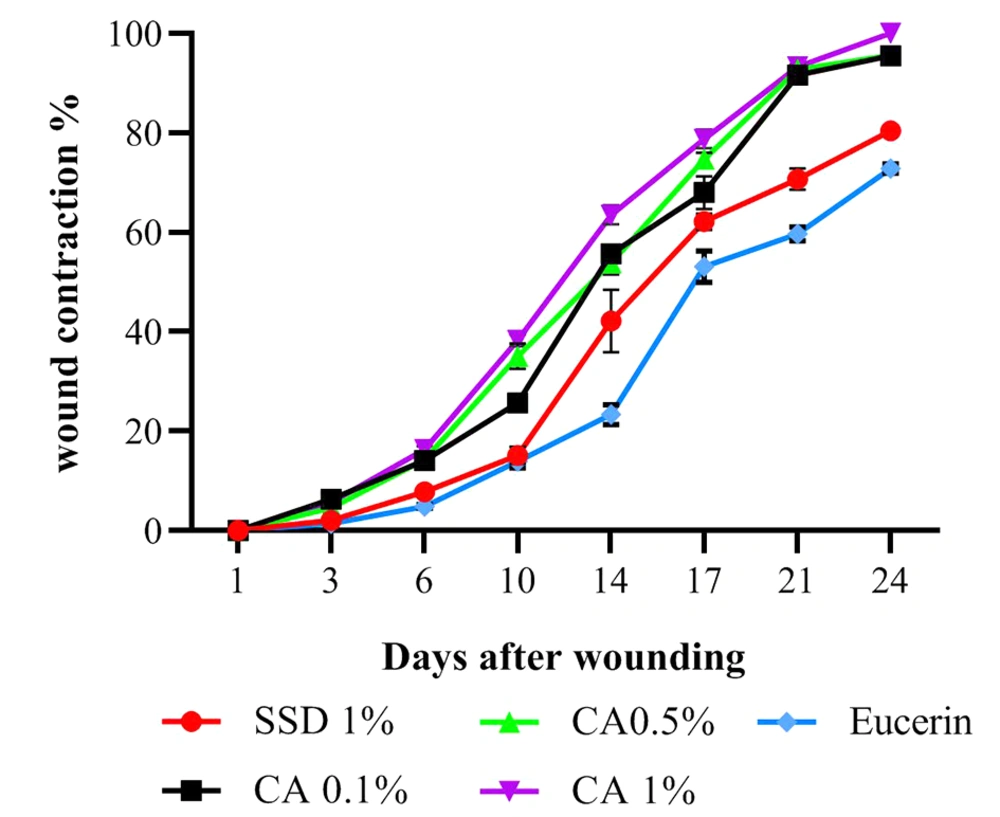
The wound contraction percentages in burn wounds treated with CA ointment at all concentrations were significantly (P < 0.001) higher than those of wounds treated with SSD and eucerin on days 6, 10, 14, 17, 21, and 24. On day 3, the wound contraction rates in CA 0.1% (P < 0.001) and CA 1% (P < 0.01) were significantly higher than those of the SSD and eucerin treated groups, but there were no significant differences among the CA 0.5%, SSD, and eucerin treated groups (Figure 1). The macroscopic views of burn wounds on days 3, 6, 14, and 24 are shown in Figure 2.
4.2. Tissue Content of Collagen
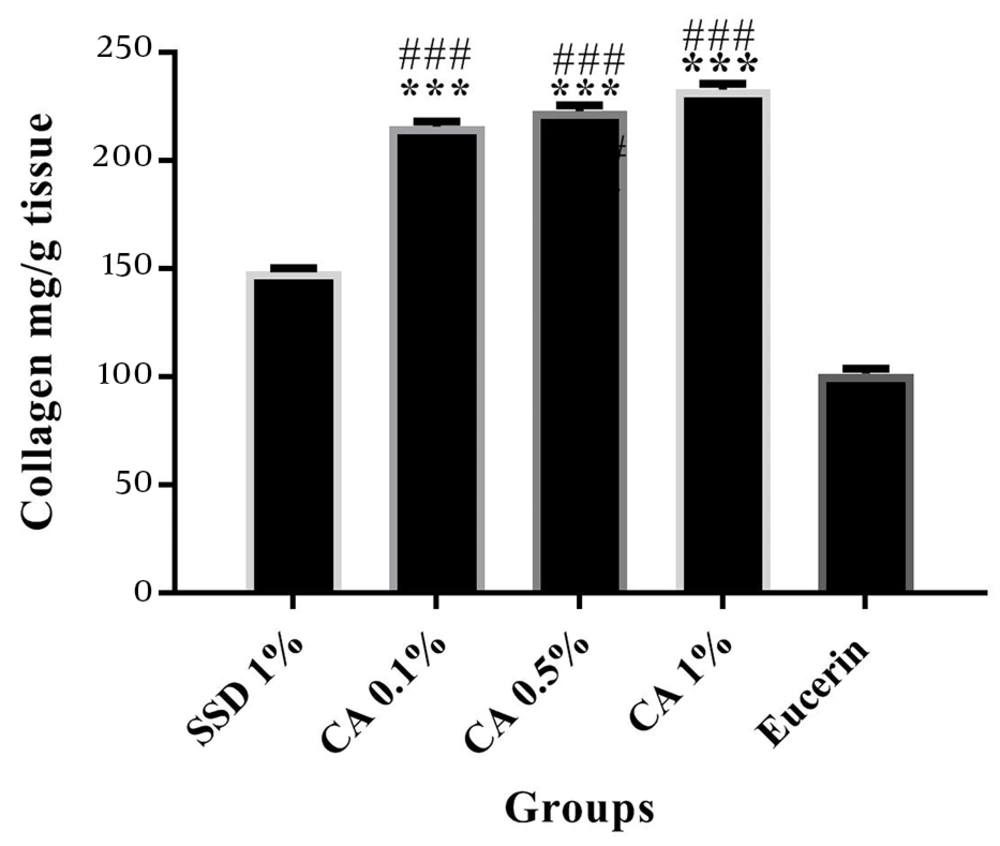
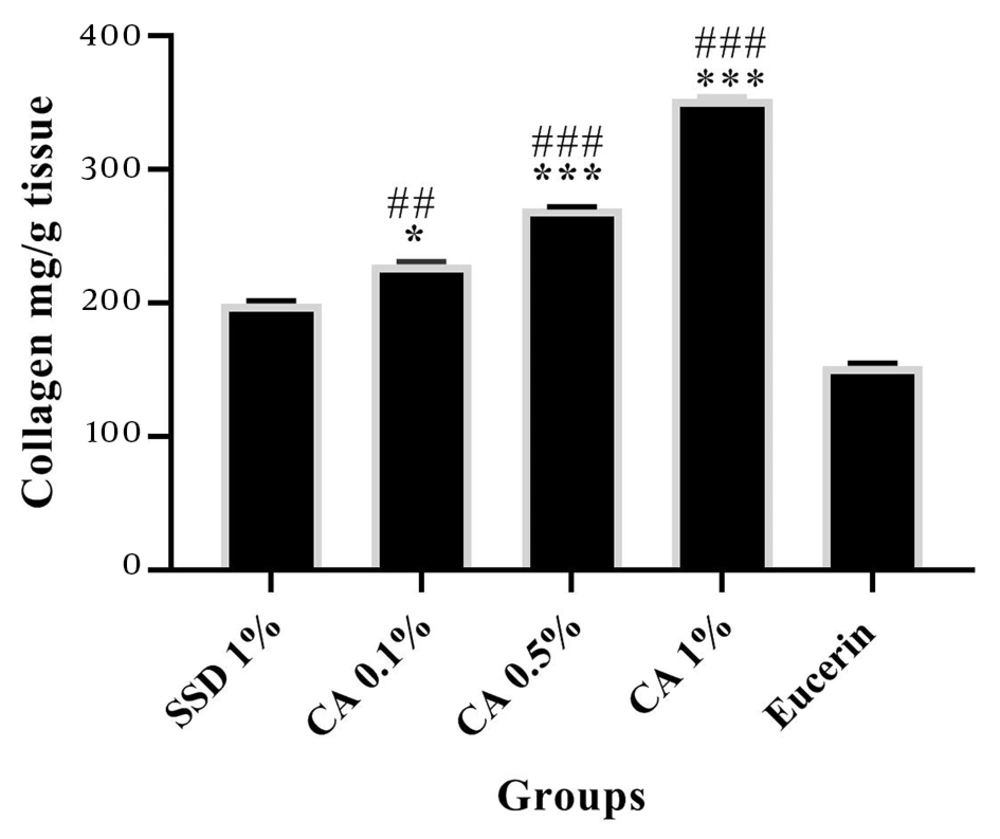
The determination of collagen content in tissue specimens on days 7 and 24 after burn wound induction revealed that the tissue content of collagen was significantly increased in the CA (at all concentrations) treated groups compared to the SSD and eucerin treated groups (Figures 3 and 4).
Comparison of collagen content of tissue samples from studied groups on day 24 post burn induction. The amounts of collagen are shown as Mean ± SEM. *** (P < 0.001) and * (P < 0.05) indicate a significant difference from eucerin, and # (P < 0.05) and ### (P < 0.001) indicate a significant difference from the SSD group.
4.3. Histopathological State of Wound Tissue in Experimental Groups
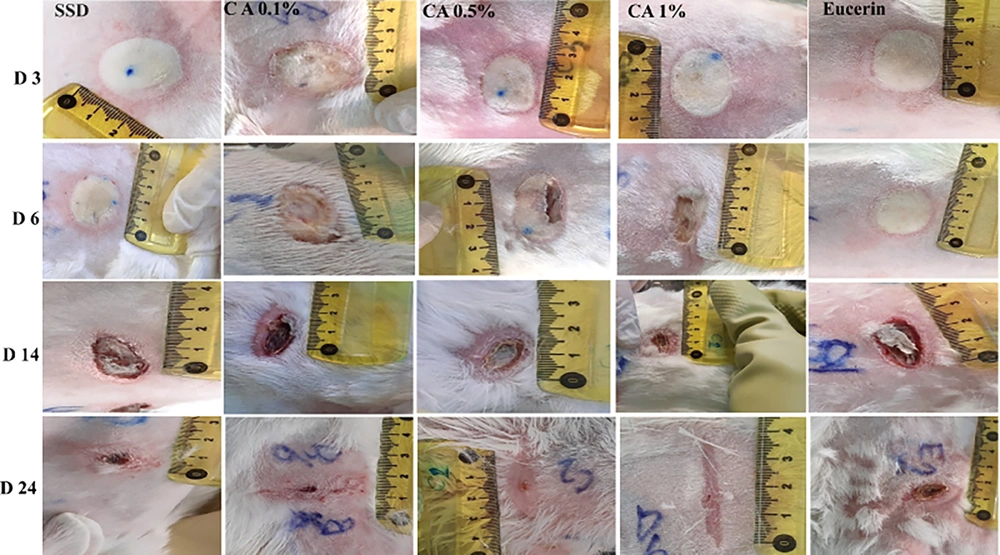
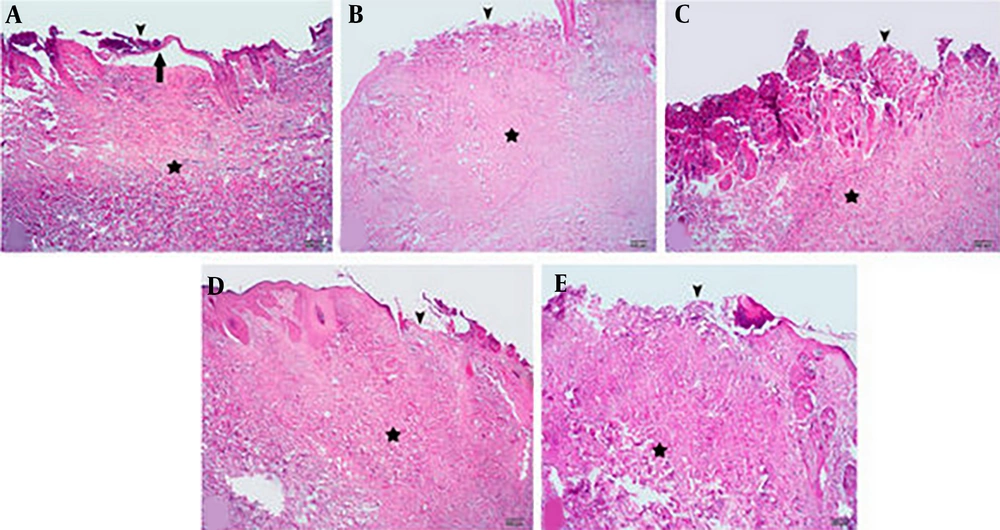
The histopathological study of specimens collected on day 7 after burn induction revealed the destruction of the epidermis and relatively severe inflammation in the dermis and hypodermis with extensive inflammatory infiltrate in tissue samples obtained from SSD, CA 0.1%, 0.5%, and eucerin groups. However, microscopic evaluation of samples obtained from CA 1% treated wounds showed the beginning of re-epithelialization, a lower degree of inflammation, and fewer inflammatory infiltrates (Figure 5).
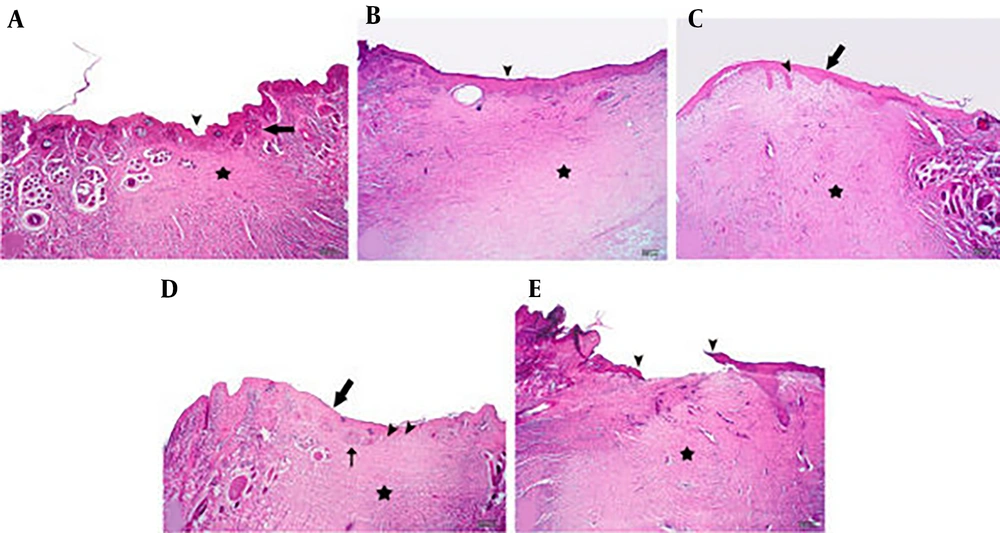
On day 24 post-wounding, in wound tissue specimens treated with CA 1%, the complete regeneration of the epidermis and the formation of dermal papillae, along with the maturation of the dermis tissue and the appearance of skin appendages, were observed. In SSD treated wounds, the epidermis was almost completely restored, and the edema and inflammation were reduced. Complete dermis regeneration, along with some degree of inflammation, was observed in CA 0.1% and 0.5% treated groups. Additionally, in CA 0.5% treated wound tissues, the appearance of papillae, indicating the beginning of the maturation of the dermis tissue, was noticed. The epidermis was not completely restored, and edema and inflammation of the dermis and epidermis were significant in samples from the eucerin treated group (Figure 6). Histopathological findings demonstrated that CA 1% had significantly better effects on the burn wound healing process compared to SSD.
5. Discussion
Burns are one of the most frequently occurring forms of skin injuries and account for a high rate of morbidity and mortality worldwide (12). Burn management remains a challenge for healthcare systems. Second-degree burns, as the most common types of burns, are associated with complications such as bacterial infection, which may lead to bacteremia and septic complications depending on the depth and extent of the burn (13). Deep second-degree burns, in which the dermis is damaged, lower the patient's quality of life and cause pain and expense. Therefore, it is essential to investigate the healing effects of potential compounds and develop safe and effective therapeutic agents to heal the deep second-degree wound rapidly (14).
The process of burn wound healing depends on the burn depth. In deep second-degree burns, healing occurs by secondary intention (where the wound cannot be sewed, causing a large extent of tissue loss), and the wound should be epithelialized and contracted. Burn wound healing occurs in three phases: Inflammation, proliferation, and maturation, similar to other types of wounds, but the duration of each phase of burn wound healing differs from that of other wound types (15). In fact, burns have a greater impact on the patient's general status (3).
Silver sulfadiazine is the most frequently used topical medicine for the treatment of burn wounds. Even though it is effective in the treatment of second-degree burns, this medicine has disadvantages, including concerns about its systemic absorption, which is significant in extensive burns. In addition, delayed wound healing has been observed following the use of topical SSD in clinical practice (16-18).
In this study, we compared the effects of ointments containing CA with SSD on deep second-degree burns in rabbits. Our findings revealed that CA accelerated the wound healing process by increasing the wound contraction rate, re-epithelialization rate, and collagen production. Previous studies indicated that CA has the potential to accelerate the wound healing process. Aquino et al. evaluated the effects of CA on fibroblast migration and concluded that CA stimulated fibroblast migration and modulated the synthesis of the extracellular matrix, indicating its potential for wound healing (19). Naghavi et al. showed the healing effects of CA on full-thickness wounds (8). These findings are consistent with our study.
The results of this study showed that CA significantly stimulated the collagen content of skin tissue. Although we did not investigate the mechanism of action of CA on burn wound healing and the increase in collagen amount, it may result from its stimulatory effect on fibroblast migration. A study by Aquino et al. showed that CA stimulated fibroblast proliferation and increased the production of collagen type І and laminin (19). Fibroblasts play a vital role in skin wound healing from the inflammation phase to the final extracellular matrix deposition, involved in processes such as the degradation of the fibrin clot and the production of cytokines, growth factors, and collagen (20).
Cinnamic acid has a high level of reactive oxygen scavenging capacity (7, 21). It has become increasingly evident that reactive oxygen species (ROS) have an intensive effect on the wound healing process by regulating processes including the inflammatory response, angiogenesis, and extracellular matrix formation (22). Reactive oxygen species scavenging by CA may contribute to its healing effects since ROS provoke local tissue damage and cause long-term inflammation (23).
Cinnamic acid is found in cinnamon (Cinnamomum cassia), grapes (Vitis vinifera), tea (Camellia sinensis), and celery (Apium graveolens), all of which have wound healing properties according to previous studies (24-27).
Infection is the main complication associated with burn wounds. The antimicrobial effect of CA is well known (7). The antibacterial and antifungal effects of both natural and synthesized CA have been reported (7). Therefore, due to its antibacterial effect, CA can prevent burn wound infection, and unlike some antibacterial agents, it not only does not cause a delay in wound healing but, on the contrary, also accelerates the wound healing process.
This study had some limitations. Although rabbits have been used as a good wound healing model for many years, the structural and physiological differences between rabbit and human skin should be considered when evaluating the translational applicability of this study.
Collectively, the results of this study demonstrate that topical cinnamic acid promoted deep second-degree burn wound healing in rabbits by increasing the collagen content of tissue and decreasing the wound closure time. Cinnamic acid could be considered as a candidate for the formulation of a safe and effective topical therapeutic agent for burn wound healing.