1. Context
1.1. Acne and Sebum Production
Acne is a prevalent inflammatory skin condition characterized by chronic inflammation of the pilosebaceous unit (1). The pathogenesis of acne involves multifaceted processes, including increased sebum production, follicular keratinization, colonization of Cutibacterium acnes, hormonal influences, skin microbiome alterations, and chronic inflammation (2). Acne typically appears on body parts with sebaceous glands, such as the face, neck, chest, upper back, and upper arms. Sebum secretion causes these areas to appear oily and shiny (3). Acne lesions begin as microcomedones, which can progress into non-inflammatory closed and open comedones, and then into inflammatory lesions like papules, pustules, and nodules (4). The chronic course of acne imposes psychological and economic burdens on patients, significantly impairing their quality of life (5).
Sebocytes located in sebaceous glands release sebum through holocrine secretion. Sebum is composed of squalene, wax esters, triglycerides, free fatty acids, cholesterol esters, and free sterols (6). It helps deliver lipid-soluble antioxidants to the skin's surface and has antimicrobial activity, making it the body's most critical barrier against external influences (7). Increased sebum production contributes to the development of acne (2). It also causes enlarged facial pores, which can be a cosmetic concern for patients without acne (8).
Although sebaceous glands were previously thought to be controlled solely by humoral mechanisms, clinical observations have suggested that they are also affected by neurological factors. A potential link between neuronal control and sebum secretion was suggested by the finding that paraplegic patients had more sebum on their thighs compared to non-paraplegic controls. Additionally, clinicians have observed changes in sebum secretion and increased acne occurrence in cases of partial facial paralysis. Furthermore, topical anticholinergics have been shown to be effective in the reduction of sebum secretion (9). In this context, several studies have also demonstrated the presence of acetylcholine (Ach) receptors on sebaceous glands (10, 11).
1.2. Botulinum Toxin Type A
Clostridium botulinum is a gram-positive, anaerobic, spore-forming bacterium that produces neurotoxins known as botulinum neurotoxins (BoNT). Serotypes of BoNTs range from botulinum toxin type A (BoNT-A) to botulinum toxin type H. BoNT-A has the ability to inhibit cholinergic neuromuscular innervation in both striated and smooth muscles, as well as cholinergic autonomic innervation in exocrine glands, depending on the specific target tissue (12). In the 1970s, it was discovered that BoNT-A had therapeutic potential, making it the most widely used BoNT in clinical applications (13). Additionally, BoNT-A was approved by the FDA in 1989 for the treatment of blepharospasm and strabismus. Further research has led to the development of new formulations and expanded the range of indications (14). In 2002, the FDA approved the use of BoNT-A for cosmetic purposes (15). As a result, BoNT-A has become widely used by neurologists and cosmetic practitioners (14).
The mechanism of action of BoNT involves inhibiting the release of acetylcholine (Ach) from motor terminals, which results in the inability of skeletal muscles to contract despite continued action potential transmission to the motor end plate. BoNT achieves this by inactivating the SNARE proteins, which are crucial for the release of Ach. The chemical paralysis of the muscles resulting from this process is reversible, with the duration of paralysis depending on the light chain half-life and SNARE protein turnover time (14).
BoNT-A is a viable option for temporarily improving moderate to severe glabellar or frown lines (15). Observations following BoNT-A application showed a significant decrease in pore size and number, as well as a reduction in skin oiliness in most patients (16). Moreover, it was observed that patients who received BoNT-A treatment for facial wrinkles experienced a significant reduction in acne, indicating a therapeutic effect on acne (17). In addition to conventional treatment methods, an increasing number of published clinical reports support the use of BoNT-A in treating acne and oily skin (18-26) (Table 1).
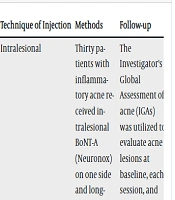
| Authors and Year | Type of Study | Object | Technique of Injection | Methods | Follow-up | Results |
|---|---|---|---|---|---|---|
| Ibrahim et al,2023 (18) | Prospective, randomized, split face | Acne improvement | Intralesional | Thirty patients with inflammatory acne received intralesional BoNT-A (Neuronox) on one side and long-pulsed Nd: YAG 1064 nm laser on the other side. The treatment was applied monthly until improvement or a maximum of three sessions. | The Investigator's Global Assessment of acne (IGAs) was utilized to evaluate acne lesions at baseline, each session, and after a 3-month follow-up. | There was a statistically significant improvement in the count of lesions and IGAs for both sides of the face. There was no statistically significant difference between both sides at the end of the treatment sessions. Facial weakness is seen in one patient. |
| Calvisi et al, 2021(19) | Prospective, uncontrolled | Acne improvement | Intradermal | Thirty-five patients with mild to moderate acne were treated with BoNT-A (Vistabex) injections. The injections were administered intradermally on the cheek in a regular grid pattern using tiny droplets (~0.01 mL, 0.2 U/cm2). | The methods of measurement used were photography, patient assessment, and Michaelsson acne scores (MAS). | Thirty-three patients, showed a significant improvement in the MAS score after treatment. A reduction in the number of papules and pustules was observed 14 days after treatment, with a significant decrease in their mean number at the end of 4 weeks. Two patients that did not respond well to the treatment. No adverse effects were observed. |
| Shirshakova et al, 2021(20) | Prospective, uncontrolled | Sebum reduction | Intradermal | Twelve patients with acne were injected intradermally with 6 - 8 U BoNT-A (no brand name) per injection area at a concentration of 0.25U/cm2on the face. | Sebum secretion was measured using a sebumeter. | There was a statistically significant reduction in skin oiliness, with the greatest effects observed two weeks after treatment. No significant side effects were reported. |
| Kesty and Goldberg, 2021(21) | Double blind, randomized, controlled | Sebum reduction | Intradermal | Fifty healthy volunteers received injections of 0, 15, 30, or 45 units of BoNT-A (Dysport) in their forehead. | Sebumeter readings, patient assessments, and investigator scores were utilized. | Patients who received either 30 or 45 U reported a significant reduction in oily skin, which persisted for 6 months. No significant side effects were observed. |
| Sapra et al, 2017(22) | Single blind, randomized, controlled | Sebum reduction, pore size tightening | Intradermal | Ten women between the ages of 35 and 65 with static wrinkles received intradermal injections of BoNT-A (Botox and Dysport) in week 0, followed by intramuscular injections in week 2. The volume of BoNT-A used for treatment was based on individual needs and applied in a regular 1 cm2 grid across the forehead and cheeks. | Sebutape, gray-scale 3D Vectra, and photographic evidence were utilized. | There was no significant effect on sebum production or pore size following intradermal BoNT-A injection. |
| Min et al, 2015 (23) | Double blind, randomized, controlled | Sebum reduction | Intramuscular | Forty-two female patients with forehead rhytids received intramuscular injections of BoNT-A (Botox) at doses of 2 or 4 units per point in five standard injection sites on the forehead. The control group was injected with saline. | Sebum secretion was measured using a Sebumeter at follow-up appointments at 2, 4, 8, and 16 weeks. | There was a statistically significant reduction in the amount of sebum secreted at the injection site. There was no statistically significant difference between the 2 U and 4 U regimens. The reduction in sebum production is most significant around 2 - 4 weeks after treatment, and levels return to normal at 16 weeks. |
| Rose and Goldberg, 2013 (24) | Prospective, uncontrolled | Sebum reduction, pore size tightening | Intradermal | Twenty-five patients between the ages of 35 and 50, consisting of 5 men and 20 women with oily skin, were intradermally injected with BoNT-A (Dysport) at a dose of 3 - 5 U per point in 10 points on the forehead. | Measurements were taken during follow-up using a sebometer, photograph and patient assessments were also recorded. | There was a statistically significant reduction in sebum at follow-up. At 1 week, the effect was greatest, and there was still a statistically significant reduction at 3 months. No significant side effects were observed except for a decrease in frontalis muscle tone in two patients. |
| Li et al, 2013 (25) | Double blind, randomized, controlled | Sebum reduction | Intradermal | The study involved 20 patients aged 21 - 37 y, 10 with oily skin and 10 with dry skin. One side of the face was injected with BoNT-A (Meditoxin) (2U/0.1 mL/cm2), while the other side was injected with a placebo (normal saline). | Sebum secretion was measured using a sebumeter and digital image analysis. | The group with oily skin showed a statistically significant reduction in sebum secretion, while no change was observed in the group with dry skin. The effect was most prevalent 4 weeks after treatment, and no significant side effects were reported. |
| Shah 2008 (26) | Retrospective, uncontrolled | Sebum reduction, pore size tightening | İntradermal | Twenty patients received intradermal injections of BoNT-A (Botox) in the T-zone, with a dosage of 0.1 mL (0.2 U/0.1 mL). | Measurements were taken during follow-up using photograph and patient assessments. | 85% of patients expressed satisfaction with the improvement in oiliness and pore size, and no visible complications were observed. |
2. Evidence Acquisition
We conducted a literature search on the PubMed, Web of Science, EMBASE, and SCOPUS databases using keywords such as 'acne,' 'acne treatment,' 'oily skin,' and 'botulinum toxin type A.' We reviewed studies that assessed the impact of BoNT-A on patients with acne and oily skin, as well as studies that measured skin sebum levels and pore size following BoNT-A application.
3. Results
3.1. Treatment Methods of Acne
3.1.1. Topical Treatment of Acne
Topical regimens for acne patients typically include combinations such as erythromycin or clindamycin with benzoyl peroxide, as well as treatments like topical retinoids, dapsone gel, azelaic acid, adapalene, and tretinoin (27).
3.1.2. Systemic Treatment of Acne
Systemic antibiotic options for acne management include oral doxycycline, minocycline, erythromycin, azithromycin, and trimethoprim/sulfamethoxazole or trimethoprim. Hormonal treatments such as spironolactone and combined oral contraceptives are also utilized. Oral isotretinoin is indicated for severe nodular acne and is also suitable for moderate acne that has not responded to other therapies or for acne that causes scarring or significant distress to the patient. Women of reproductive age should receive information about contraception prior to initiating isotretinoin treatment. It is recommended to perform baseline liver function tests, as well as serum cholesterol and triglyceride measurements initially, and to repeat these tests during the course of treatment (27).
3.1.3. Physical Treatment Methods of Acne
Physical treatments, such as chemical peels (e.g., salicylic acid, mandelic acid, Jessner’s peel) and light therapies (including photochemical therapies such as blue, red, or combined blue/red light, and photodynamic therapy, which involves a light source such as red light, blue light, or daylight, along with a photosensitizing chemical such as 5-aminolaevulinic acid or methyl aminolaevulinate), are other modalities used for acne treatment (28).
3.2. Acne, Sebum Production and Botulinum Toxin Type A
The use of BoNT-A has expanded to various areas in dermatology. While it is commonly used for cosmetic purposes, it has also been shown to improve enlarged pores, scars, and oily skin (29). In a recent study, BoNT-A was used to treat patients with inflammatory acne. This prospective randomized split-face comparative study aimed to compare the clinical efficacy and safety of long-pulsed Nd:YAG laser 1064 nm with intralesional BoNT-A for managing inflammatory acne. Thirty patients with inflammatory acne were treated with the long-pulsed neodymium: Yttrium-aluminum-garnet (Nd:YAG) laser 1064 nm on one side of their faces and intralesional BoNT-A on the other side, monthly, for a maximum of three sessions, until improvement was observed. Acne cases were evaluated by counting lesions and grading severity using the Investigator's Global Assessment of Acne (IGA) at baseline, each session, and after a 3-month follow-up. A dose of 2.5 units (U) of BoNT-A (Neuronox®, Medytox Inc., Gangnam-gu, Seoul, South Korea) was injected per 0.1 ml intradermally directly into acne lesions, with 1 to 3 U of BoNT-A per lesion. The maximum dose used per session was 20 U, and the distance between adjacent injection points was 2 to 3 cm. A statistically significant improvement in the number of lesions and IGA scores was recorded for both sides, with a statistically non-significant difference between the two sides at the end of the treatment period. After a 3-month follow-up, a more significant improvement was observed on the laser therapy side. In conclusion, both long-pulsed Nd:YAG laser 1064 nm and intralesional BoNT-A were found to be safe and effective for acne therapy. However, it should be noted that intralesional BoNT-A was found to be less effective and had a higher recurrence rate than Nd:YAG laser 1064 nm. A side effect was observed in one patient after the second session, manifesting as weakness in the facial muscles (18). Another study aimed to treat patients with acne and rosacea using BoNT-A. A total of 35 patients with mild to moderate acne were treated with intradermal injections of BoNT-A (Vistabex®, Allergan S.p.a., Via Salvatore Quasimodo, 134 - 138, 00144 Roma) on the cheek in a regular grid pattern using very small droplets (~0.01 mL, 0.2 U/cm²). After treatment, 33 patients exhibited a significant (> 55.4%) improvement in the Michaelsson acne scores and a 3.6 rating in the Subject Global Aesthetic Improvement Scale, while two did not respond effectively to treatment. A reduction in the number of papules and pustules was observed 14 days after treatment, with a significant decrease in the mean number of papules and pustules at the end of 4 weeks. No adverse effects were reported in the study (19). In one study assessing changes in facial skin seborrhea and enlarged pores, a multifocal intradermal technique was used evenly over the entire facial area of 12 patients at a dosage of 0.25 U/cm² or 0.125 U/0.5 cm² of an unidentified brand of BoNT-A formulation. The study reported a significant reduction (P ≤ 0.01) in sebum secretion and pore diameter with BoNT-A treatment compared to baseline levels. No side effects were observed in the study (20). A double-blind, randomized, placebo-controlled study was conducted to assess the efficacy of BoNT-A injections for treating oily skin on the forehead. The study involved 50 male and female subjects who received intradermal injections of a randomized number of units of BoNT-A (Dysport®, Galderma Laboratories, L.P., Fort Worth, TX) in their forehead. The doses of BoNT-A administered were 0, 15, 30, or 45 U. Both researchers and subjects reported a significant decrease in forehead oiliness after receiving at least 30 U of BoNT-A (P < 0.05). Additionally, subjects who received 30 or 45 U of BoNT-A had significantly lower sebometer counts compared to both the placebo group and subjects treated with 15 U of BoNT-A (P < 0.05). This effect persisted at the 6-month follow-up visit. No adverse events related to treatment were observed during the study (21). Another study, a single-blind, split-face, randomized pilot study, involved ten women aged between 35 and 65 with static wrinkles in the glabellar and periorbital areas. The study aimed to evaluate the difference in the effect of intradermal and intramuscular application of two commercial BoNT-A products for treatment. The products were administered based on individual requirements and applied in a consistent 1 cm² pattern across the forehead and cheeks during week 0, followed by intramuscular injections in week 2. However, intradermal injection of BoNT-A did not result in a significant reduction in pore size or sebum production among patients (22). A prospective randomized double-blind study was conducted on 42 female patients with forehead wrinkles. They were injected with BoNT-A (Botox®, Allergan, Irvine, CA) intramuscularly. The study compared the effects of administering 2 U versus 4 U of BoNT-A in the forehead region. A total of 10 to 20 U of BoNT-A was administered intramuscularly as a treatment. The administration of BoNT-A through intramuscular injection significantly reduced sebum production compared to baseline measurements. This effect was not dependent on the dosage of the treatment. After 16 weeks, sebum production levels returned to their original levels (23). Another study evaluated the efficacy and safety of BoNT-A for oily skin in 5 male and 20 female subjects with mild to moderate forehead oiliness. A total of 30 - 45 U of BoNT-A (Dysport®, Medicis, Scottsdale, AZ) were injected intradermally into the frontalis muscle region at 10 points, with 3 - 5 U at each point. Sebum production significantly decreased at all follow-up time points assessed (P < 0.001), as measured by the sebometer. The patients' satisfaction rate was 91% based on their evaluations. Two subjects exhibited a decrease in frontalis muscle tone two months after treatment. No additional side effects were observed in the study (24). Li et al. conducted a double-blind, placebo-controlled, split-face study involving twenty healthy volunteers aged 21 - 37 years. One side of each participant's face was injected intradermally with 2 U/cm² of BoNT-A (Meditoxin®, Medytox, Seoul, Korea) at four points, while the control side received normal saline injections. Among the participants, ten were categorized as having oily skin, while the remaining had dry to normal skin. After the intradermal injection of BoNT-A, the oily skin group exhibited a statistically significant reduction in pore size and a marked decrease in sebum production after 4 weeks. No significant adverse effects were detected throughout the study (25). A retrospective study assessed the safety and effectiveness of a single intradermal injection of BoNT-A in reducing pore size and sebum excretion on the forehead, nose, and chin in 20 patients. One month after the injection, 17 out of 20 patients reported a decrease in pore size and sebum production. The patients expressed satisfaction with the procedure and did not experience any complications (26).
3.3. Physiologic Effects of Botulinum Toxin Type A on Acne and Sebum Production
Non-neuronal cells throughout the body express a major subunit of nicotinic acetylcholine receptors (nAchR), known as nAchRa7 (30). The sebaceous gland prominently expresses distinct nAchR subunits, including nAchRa7, and releases acetylcholine (Ach) through autocrine mechanisms (10, 11). The inhibitory effect of α-bungarotoxin on Ach-induced lipid synthesis supports the conclusion that nAchRa7 expression in human sebocytes plays a role in this process. Therefore, it has been demonstrated that Ach signaling increases sebum production (25). Moreover, in vitro findings have shown that oily skin may be more susceptible to cholinergic modulation compared to normal skin due to the higher number of mature sebocytes with increased cholinergic receptors (25).
Contraction of the arrector pili muscles can significantly affect sebum excretion (31). Li et al. demonstrated that cholinergic signaling impacts sebocytes and sebum production and that BoNT-A reduces sebum production (25). Additionally, it is predicted that BoNT-A activity affects the arrector pili muscle, which in turn influences sebum production and pore size (24).
In the treatment of inflammatory acne, BoNT-A is proposed to block Ach at the synaptic cleft, thereby inhibiting cholinergic innervation of the sebaceous gland and ultimately reducing sebum production (25). In addition, local muscarinic receptors and arrector pili muscles are inhibited (24), resulting in reduced sebum production and minimized pore size. Furthermore, BoNT-A suppresses neurogenic inflammation by inhibiting substance P, tumor necrosis factor-α, calcitonin gene-related peptide, and neurokinin (20, 32). Therefore, BoNT-A has the potential to benefit acne by reducing inflammation, a key component of acne pathogenesis.
The studies indicate that BoNT-A can effectively reduce sebum secretion and skin oiliness when administered in appropriate dosages. Two studies on acne treatment showed that BoNT-A was effective when applied intralesionally or intradermally (18, 19). Both intradermal and intramuscular routes were used in the studies to reduce sebum secretion. According to Min et al., the intradermal method may be more efficacious (23). Calvisi et al. suggested that "microbotox," a specific dilution of intradermal BoNT-A, may be effective for treating mild-to-moderate acne vulgaris (19). However, Sapra et al.'s study found that both intradermal and intramuscular injection methods of BoNT-A were ineffective in reducing pore size and sebum production (22).
Ibrahim et al. reported that the use of higher dilutions of BoNT-A results in greater diffusion areas, leading to a higher incidence of adverse effects. Conversely, the authors noted that weakness in facial muscles caused by intradermal injection of BoNT-A was temporary and reversible. The potentially shorter duration of the toxin's effect with superficial injection of BoNT-A, as opposed to intramuscular injection, is due to it only blocking the superficial facial and neck expression muscles (18). Calvisi et al. discussed the importance of precisely targeting intradermal structures, such as the sebaceous gland, with BoNT-A. They emphasized that injecting the toxin too superficially would not be effective, while injecting it too deeply could lead to inadvertent paralysis of the underlying skeletal muscles (19).
Currently, there is no conclusive data indicating a correlation between excessive or insufficient dosage of BoNT-A and sebum production. Kesty and Goldberg suggested that higher doses of BoNT-A are more effective than lower doses in reducing sebum production (21), but Min et al. indicated that the effect of BoNT-A was not dependent on the dosage (23). Additionally, the efficacy duration varied among the studies, which may be influenced by the dosage and brand of the BoNT-A product (19, 33).
4. Conclusions
Studies evaluating the management of acne and reduction of sebum with BoNT-A treatment have shown variations in dosage and duration of effectiveness. As the effects of BoNT-A are reversible, oily skin and acne may reappear when the efficacy diminishes. Furthermore, the chronic nature of acne requires repetitive treatment, which may pose a challenge for patients, especially from an economic perspective. However, BoNT-A exhibits potential as a treatment for acne and oily skin, with intradermal applications showing effectiveness and minimal side effects. This introduces a new method for addressing acne and oily skin, which could significantly impact the future of acne treatment. Further studies are needed to determine the optimal treatment method for acne and oily skin due to the diversity of concentrations, dosages, and duration of efficacy of BoNT-A.