1. Background
Cutaneous leishmaniasis (CL) is still a significant health problem in Africa, Asia, and Latin America. According to the World Health Organization (WHO) report, 88 countries are afflicted with CL, and about 90% of the affected patients live in the following seven countries: Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria (1). One of the most important foci of the CL in Iran is Isfahan (2). Numerous treatments with variable efficacies have been suggested for CL treatment. In the last few years, resistance to meglumine antimoniate (MA) has been increased (3, 4). Therefore, medications with minimal side effects and optimum efficacy have been sought. Herbal drugs might have important roles in this respect. One of the plants used with variable success rates for CL treatment in southern parts of Iran is Cassia fistula (5). C. fistula has a wide variety of uses in folk medicine including treatment of diabetes, diarrhea, dysentery, fever, and uterine or menstrual disorders (6, 7). In addition, antiviral and hepato-protective activities have been previously reported (6). Acetone soluble fraction of this plant seeds has a bioactive glycoside with antimicrobial activity (7). C. fistula extract has been used for eradication of some bacteria such as Escherichia coli, Klebsiella, Staphylococcus aureus, Proteus vulgaris, and Pseudomonas aurogenosa (8). Antioxidative effects were also reported for this extract, attributed to its phenolic and flavonoid compounds, and proanthocyanidin (9). Sartorelli et al. reported significant antileishmanial activity of C. fistula fruits hexane extracts on Leishmania chagasi (10). Mohebali et al. reported that 25% C. fistula extract could be effective in CL treatment (11).
2. Objectives
This study was designed to assess the effectiveness of concentrated boiled extract and hydroalcoholic extract of C. fistula on leishmaniasis lesions in comparison with intralesional injection of MA.
3. Materials and Methods
This study was a randomized parallel clinical trial for CL treatment, conducted in the Skin Diseases and Leishmaniasis Research Center (SDLRC), Isfahan, Iran. The institutional review boards of SDLRC approved the study protocol and all patients signed a written informed consent before entering the study. A total of 165 patients with confirmed CL, 6-60 years old, were recruited. CL was confirmed by examining the direct smear of the lesions. Demographic characteristics of the patients are shown in Table 1. Patients with the following criteria were excluded from this study:
1) The size of lesion was more than 3 cm.
2) The number of lesions was more than five.
3) The lesion duration was more than 12 weeks.
4) The lesion was located on the eyelid or less than 2 cm away from the eye.
5) Pregnant or lactating patients.
The patients were randomly allocated to three groups using table of random numbers. First group was treated with concentrated boiled extract of C. fistula, second group with its hydroalcoholic extract, and third group by intralesional injection of MA. The first applications of hydroalcoholic and concentrated boiled extracts of C. fistula were performed by the physicians. Thereafter, patients were instructed to apply the extract-soaked gauze on the lesions once a day until complete resolution of the lesion (complete epithelialization) or for a maximum duration of four weeks. The third group was treated by intralesional injection of MA (0.5-2 mL) twice a week until complete resolution of the lesion or for a maximum duration of four weeks. In all patients, size of the lesion was measured at weekly intervals for the first four executive weeks and at week 16, using glassy paper and millimeter paper for measuring the surface area of the lesion. At the end of the study, direct smear and culture were repeated. In the case of lesion progression or development of new lesions during the treatment, or patients’ reluctance to continue the treatment, they were excluded from the study and their treatments were followed with other appropriate medications. Lesions were considered completely cured (improvement), if the patients achieved both clinical and parasitological resolutions (negative results of direct smear). Patients were considered resistant to the treatment, if no clinical changes were observed in the lesion or it was worsened. All patients who received at least one dose of the study treatment were included in the final analyses. All statistical tests were two-sided with significance (α) level of 0.05. The treatment groups were compared using chi-square and ANOVA tests. The data were analyzed using SPSS 10 software (SPSS Inc., Chicago, Illinois, USA).
3.1. Preparation of Cassia fistula Extracts
3.1.1. Preparation of Hydroalcoholic Extract
Five hundred grams of C. fistula was powdered and mixed with 70% ethanol with ratio of 1:3, then placed in a percolator. After 48 hours, extraction was performed. The extract was then concentrated using distillation in vacuum condition.
3.1.2. Preparation of Concentrated Boiled Extract
Five hundred grams of C. fistula was powdered and then mixed with water with ratio of 1:3, then boiled for 30 minutes and concentrated by distillation in vacuum condition.
4. Results
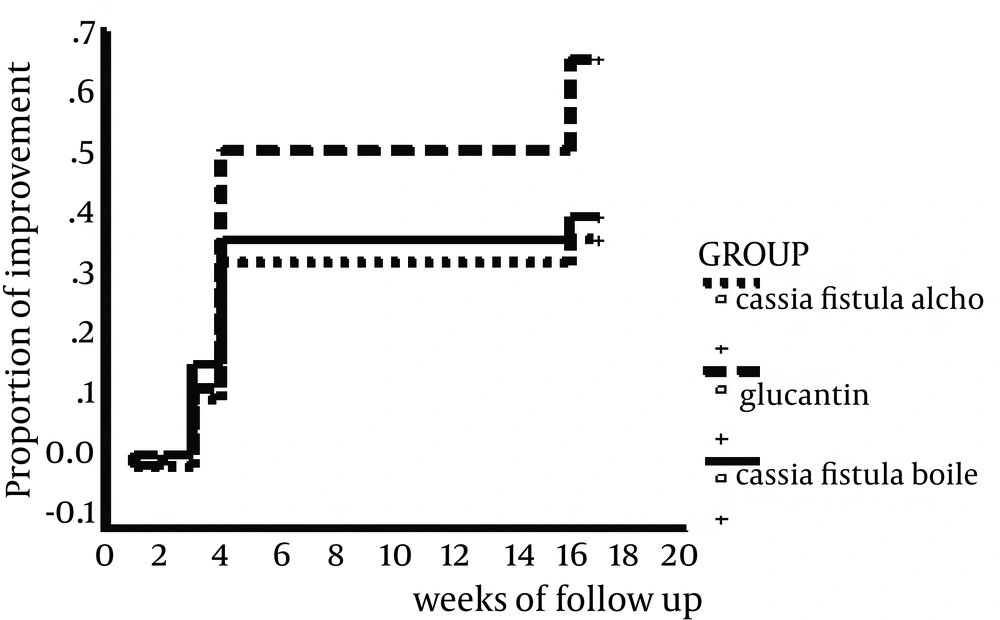
Demographic characteristics of the patients are shown in Table 1. Complete improvements were observed in 22 (40%), 20 (36.4%) and 36 (65.5%) patients in concentrated boiled extract of C. fistula, hydroalcoholic extract of C. fistula, and intralesional injection of MA, respectively. Efficacy of MA intralesional injection was significantly higher than concentrated boiled extract (P = 0.02) and hydroalcoholic extract of the C. fistula (P = 0.005) applications. There was no significant difference between application of concentrated boiled extract and hydroalcoholic extract of C. fistula regarding the efficacy (P = 0.61). The mean resolution time was 4.6 ± 3.7, 4.9 ± 3.8, and 6.4 ± 5.2 weeks for concentrated boiled extract application, hydroalcoholic extract application, and intralesional injection of MA, respectively. There was no statistically significant difference among the groups regarding the resolution time (P = 0.27). In the present study, 3 (5.5%) patients were in the concentrated boiled extract group and 2 (3.6 %) patients in the other groups withdrew from the study due to the allergic reaction to the medications. There was no significant difference among these three groups in this regard (P = 0.61).
| Concentrated Boiled Extract of C. fistula | Hydroalcoholic Extract of C. fistula | MA | P Value | |
|---|---|---|---|---|
| Gender | 0.15 | |||
| Male | 35 (63.6) | 29 (52.7) | 25 (45.5) | |
| Female | 20 (36.4) | 26 (47.3) | 30 (54.5) | |
| Age, y | 20.6 ± 12.4 | 19.8 ± 11.5 | 22.9 ± 13.6 | 0.4 |
| Location of lesions | 0.2 | |||
| Foot | 9 (16.4) | 11 (20) | 8 (14.5) | |
| Hand | 25 (45.5) | 23 (41.8) | 24 (43.6) | |
| Trunk | 5 (9.1) | 6 (11) | 5 (9.1) | |
| Head and neck | 8 (14.5) | 10 (18.2) | 11 (20) | |
| Hand and foot | 8 (14.5) | 5 (9.1) | 7 (12.7) | |
| Number of lesions | 1.8 ± 1.13 | 1.72 ± 0.98 | 2.03 ± 1.07 | 0.3 |
| Kind of lesions | 0.02 | |||
| Nodule | 23 (41.8) | 30 (54.5) | 39 (70.9) | |
| Papule | 17 (30.9) | 11 (20) | 5 (9.1) | |
| Papule and nodule | 15 (27.3) | 14 (25.5) | 11 (20) |
a Data are presented in Mean ± SD or No. (%).
bAbbreviation: MA, Meglumine Antimoniate
There was also no significant difference in the response rates among these three groups during the first four executive weeks of the treatment (P = 0.4) (Table 2). Cumulative proportions of the improvement in these three groups during the study are presented in Figure 1.
a P = 0.02 compared with MA.
b P = 0.005 compared with MA.
5. Discussion
Given many side effects of antimony compounds, there have been several trials for developing new alternative treatment options for CL. Among these trials, many treatments have been based on herbal remedies to find an effective topical modality for CL. Chauhan et al. reported antileishmanial effect of C. fistula hexane extract on L. chagasi promastigotes (12). Moreover, the C. fistula fruit gel has had an additive effect along with the intralesional MA injection in treating acute CL by increasing the resolution rate of the lesions (13). This study showed that intralesional MA injection was more effective than applying hydroalcoholic or concentrated boiled extracts of C. fistula. The healing time was shorter in treatment with hydroalcoholic as well as concentrated boiled extracts compared with intralesional MA injection. Although even doses as high as 20000 g of this plant were shown to be nontoxic, there have been a few studies regarding the efficacy of this plant in CL treatment (14). C. fistula showed significantly better wound closure and tissue regeneration in treated rats compared with the controls (15). This evidence could be contributed to its efficacy in healing CL lesions. Our study showed that almost 40% of the patients treated with this plant achieved complete resolution of the lesions. This finding offers clinical evidence on the folkloric use of C. fistula extracts for CL treatment. We recommend further evaluation of concurrent topical use of this plant with MA injection, to hasten the resolution of leishmaniasis ulcer, and possibly decrease the dose of applied MA. A complete phytochemical analysis is required to identify the active ingredients, with potential contribution in the observed effectiveness of C. fistula in CL treatment.