1. Context
Stem cells were first studied in 1963, when bone marrow cells were injected into irradiated mice and noticed tumors growth in mice spleens. They concluded that any nodule arose from a single bone marrow cell. In addition, they found that these cells were capable of self-renewal. There are two main types of stem cells, embryonic and non-embryonic types. Embryonic stem cells are totipotent and can differentiate into all three embryonic germ layers and non-embryonic stem cells, known as adult stem cells, are multipotent and their ability to differentiate into different cell types assumed to be more limited (1).
2. Embryonic State
2.1. Embryonic Stem Cells
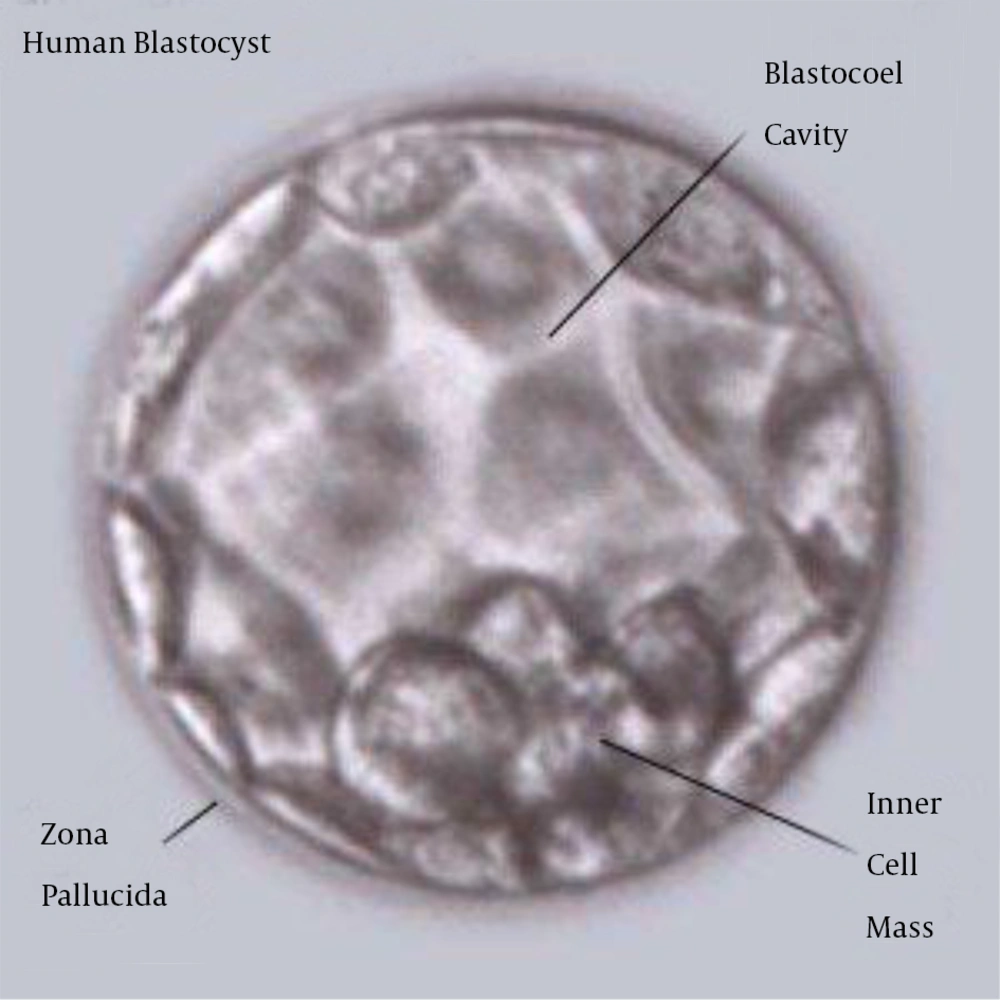
Human embryonic stem cells (ES cells) are undifferentiated cells that have self-renewal and differentiate into all cell types. The derivation of ES cells from mouse was first reported in 1981 and in 1998 the derivations of human ES cell lines were reported. Embryonic stem cells are isolated from inner cell mass (ICM) of embryo. ICM cells have the potential to generate any cell type, but after implantation, these cells differentiate to other cell types. ES cells can be isolated by immunosurgery from the inner cell mass of embryo during the blastocyst phase and are usually grown on feeder layers consisting of embryonic fibroblast cells. Recent reports showed that these cells can be grown without using a feeder layer. These cells have demonstrated longevity in culture by maintaining their undifferentiated state for 80 passages. Recently studies showed that human hESCs differentiate into keratinocytes, chondrocyte and osteocyte (2-4) (Figure 1).
2.2. Embryonic Germ Cells
In 1998 isolation, culture and characterization of germ cells derived from the gonadal ridge of human tissue obtained were shown. Embryonic germ (EG) cells are derived from primordial germ line cells in primitive fetal tissue. The range of potential fates would be relatively limited compared to embryonic stem cells, because EG cells are much further along in evolvement (5-9 weeks). Fetal tissue may provide deposited progenitors, but the possibility of large scale sourcing and construction of products using such cells is doubtful. Furthermore, behavior of these cells in vivo is not well understood; much research would be required to avoid unwanted outcomes, including ectopic tissue formation, tumor induction or other unusual development (5, 6).
2.3. Amniotic Epithelial Cells
Amniotic epithelial cells (AECs) are derived from the amniotic membrane in placenta. These cells express the markers present in pluripotent embryonic stem cells and embryonic germ cells, such as Oct-4, Nanog and alkaline phosphatase (AP). Moreover, these cells differentiate as ESCs and EGCs in the cell lineages from three germ layers. Because of the observation that they proliferate at a high rate without apparent loss of pluripotency or teratogenic potential when transplanted in immunodeficient animals, amniotic fluid stem cells were credited with being a safer alternative to hESCs (7, 8).
2.4. Amniotic Fluid-Derived Stem Cells
In 2007, isolation of multipotent stem cells from amniotic liquid was performed. These undifferentiated cells were found to express some embryonic stem cell markers. These cells show a mediocre stage between the two types of stem cells, ESCs and non-ESCs. Amniotic fluid-derived stem cells were found to expand extensively. This result was obtained without the need to use a feeder layer, doubling and retaining long telomeres to over 250 population doublings. These cells did form teratomas in vivo. These cells have the capability to differentiate into functional cells corresponding to any of the three main embryonic germ layers. These cells were able to give rise to adipogenic, osteogenic, myogenic, endothelial, neuronal and hepatic cells. The ability to derivation genetically and phenotypically constant pluripotent cells from such a widely and easily available source would potentially have an emphasis on regenerative therapy in the future (9).
2.5. Umbilical Cord Blood Stem Cells
In 1980s, umbilical cord blood stem cells were recognized as an important source of HSCS. Blood from the placenta and umbilical cord is a valuable source of hematopoietic stem cells. Cord blood stem cell technology has many advantages over embryonic and other adult stem cells for several reasons including; (A) cord blood displays a potentially unlimited source of stem cells that can in theory be gathered at every birth; (B) cord blood is relatively simple to process and store using tried and tested technology and is biologically stable once frozen in liquid nitrogen; (C) collection of cord blood is a noninvasive method. If cord blood is not collected, it is discarded as biological waste; and (D) cord blood carries low risk of infection (10, 11).
3. Non Embryonic State
3.1. Bone Marrow Stem Cells
3.1.1. Bone Marrow Hematopoietic Stem Cells
Hematopoietic stem cells have specific morphological appearances and cell-surface markers that allow them to be labeled and tracked in the blood flow and goal tissues or to be isolated and cultured in laboratory. Hematopoietic stem cells represent less than 0.05% of the whole bone marrow, but have the potential to reconstitute all blood forming lineages (8).
3.1.2. Bone Marrow Stromal Stem Cells
Scientists described the marrow stroma as a heterogeneous population of connective tissue cells. These cells support structurally marrow hematopoiesis. This structure of heterogeneous cells constitutes the niche of haematopoietic stem cells, supporting bone marrow cells. The term bone marrow stromal cell (BMSCs) was applied to isolated bone marrow cells with potential to form connective tissues. Among these BMSCs, there is a subpopulation of undifferentiated multipotent cells able to generate the mesenchyme. These cells present in all postnatal tissues and is defined as mesenchymal stem cells. These cells differentiated into multiple mesenchymal cell lineages in laboratory, including bone, ligament, adipose, cartilage and muscle. BMSCs or MSCs are usually isolated from the mononuclear layer of bone marrow after separation by density gradient centrifugation. These mononuclear cells are cultured in culture media containing 10-15% fetal calf or autologous serum. BMSCs adhere to the tissue culture plastic, leaving small adherent fibroblast-like cells. These cells divide and proliferate rapidly (12, 13).
3.2. Multipotent Adult Progenitor Cells
The adult bone marrow has a heterologous population of stem cells, named multipotent adult progenitor cells and organize a population of stem cells isolated from or closely related to embryonic stem cells (14).
3.3. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are capable of self-renewal and differentiation into multiple cells. MSCs are multipotent and easily derived from a variety of tissues, containing fat, skin and bone marrow. Now, bone marrow and gingiva are considered as prime sources of MSCs. These cells are fibroblastic in appearance and can be populated for many passages. Populations of mesenchymal stem cells are strongly adherent. Mesenchymal stem cells can give rise to many kinds of connective tissue cells containing those responsible for remodeling of cartilage, bone, fat and vascular tissue. MSCs can be derived from circulating blood, as well as diverse nonhematopoietic tissues such as synovium, adipose tissue, trabecular bone, dermis, dental pulp and lung. Human adult mesenchymal stem cells are non-hematopoietic, adherent fibroblast-like cells with capacity of self-renewal and potential for multilineage differentiation. Isolated MSCs present surface markers such as Thy-1 (CD90), SH-2/endoglin (CD105), SH-3/4 (CD73), b-1-integrin (CD29) and CD44 and are negative for hematopoietic markers such as CD34, CD14 and CD45 (15, 16).
3.4. Neural Stem Cells
Neural stem cells can self-renew and have the capacity to generate glial and neuronal lineages. Neuronal stem cells have been isolated from the brains of embryos and adults. Adult neural stem cells have the potential to differentiate into cell types of the brain, mainly oligodendrocytes, astrocytes and neurons and used in transplants for Parkinson’s disease. These cells may be multipotent. In brain-injury models, neural stem cells (NSCs) proliferate in those neurogenic regions and migrate toward the site of damage (17).
3.5. Pancreatic Stem Cells
The mammalian adult pancreas has three tissue types; the ductal tree, the exocrine acini and the endocrine islets of Langerhans. Multipotent cell progenitors have been recognized within the ducts and islets in adult rodent and human pancreas. Pancreatic stem cells (PSCs) were derived from human fetal pancreas, which present stem cell markers nestin, ATP binding cassette transporter (ABCG2) and KIT (18).
3.6. Skin Stem Cells
Skin is the largest organ in the body. Skin is derived from the embryonic ectoderm germ layer. In 2001, isolation of multipotent cells from the dermis was reported. These cells proliferated and differentiated in culture to produce both neural and mesodermal cells, including neurones, glia, smooth muscle and adipocytes. Nestin-positive, skin-derived precursors (SKPs) are a different type of stem cells. Most neural cells generated by SKPs are similar to peripheral neurones and Schwann cells. Thus, SKPs represent an embryonic neural crest-related precursor. SKPs can be derived from human tissue, including as little as 1-2 cm of foreskin sample and small punch biopsies from the scalp. SKPs are an available source of neural precursors, which can be used for regenerative medicine of the nervous system. Some studies have shown that the upper region of hair follicles, the bulge area, constitutes the niche of multipotent stem cells, which are responsible for long-term growth of hair follicles and epidermis regeneration after tissue injury. More specifically, multipotent epithelial stem cells (bESCs) within the bulge area, which express CD34, K5 and a 6 integrin, are able to proliferate and give rise to the follicular epithelium, as well as new cells constituting IFE and sebaceous glands after severe injury. The bulge area in adult mammalian hair follicle also contains a pluripotent epidermal neural crest stem cell (eNCSC) population that shows several properties similar to embryonic neural crest stem cells. The pluripotent eNCSCs in the bulge area are also able to self-renewal and give rise to multiple cells in vivo, including melanocytes, neurons, Schwann cells, smooth muscle cells and chondrocytes (19-21).
3.7. Cardiac Stem Cells
Cardiac stem cells are undifferentiated and present in the adult heart of mammals. These cells mediate endogenous mechanisms for repair and substitution of ongoing cells within the adult heart. Cardiac progenitor cells are multipotent and give rise to cardiomyocytes and coronary vessels in vivo, such possessing the substantial properties of stem cells. Cardiac stem cells can be isolated from patients and cultured ex vivo to generate great numbers of cells. A recent report demonstrated that cardiac stem cells increase in number after MI, but in the chronic step, the numbers fall and the residual cardiac stem cells have less regenerative capacity. There are no clinical trials of human cardiac stem cells (22, 23).
3.8. Fat Stem Cells
Human adipose tissue is a source of multipotent stem cells. Adipose derived stem cells (ADSCs) can be induced to differentiate in vitro into various other cell lines, including chondrogenic, adipogenic, osteogenic, hepatic and neurogenic lineages. Studies showed that phenotype and genotype of these cells at a transcriptional level are fully like to those of BMSCs.
ADSCs are a good source for regenerative medicine and can be maintained for long periods with population doublings and low senescence. Although the bone marrow is a good source of stem cells, its derivation is an invasive method and the number of derived cells can be low and age dependent. In addition, only 0.01-0.001% of mononuclear cells derived from the bone marrow lead to colony-forming units, but adipose tissue can yield large amounts of stem cells and obtained in abundance (24-26).
3.9. Olfactory Adult Stem Cells
Olfactory adult stem cells have been isolated from the human olfactory mucosa cells, the lining of nose involved in the feeling of olfaction (27, 28) (Figure 2).
4. Induced Pluripotent Stem Cells
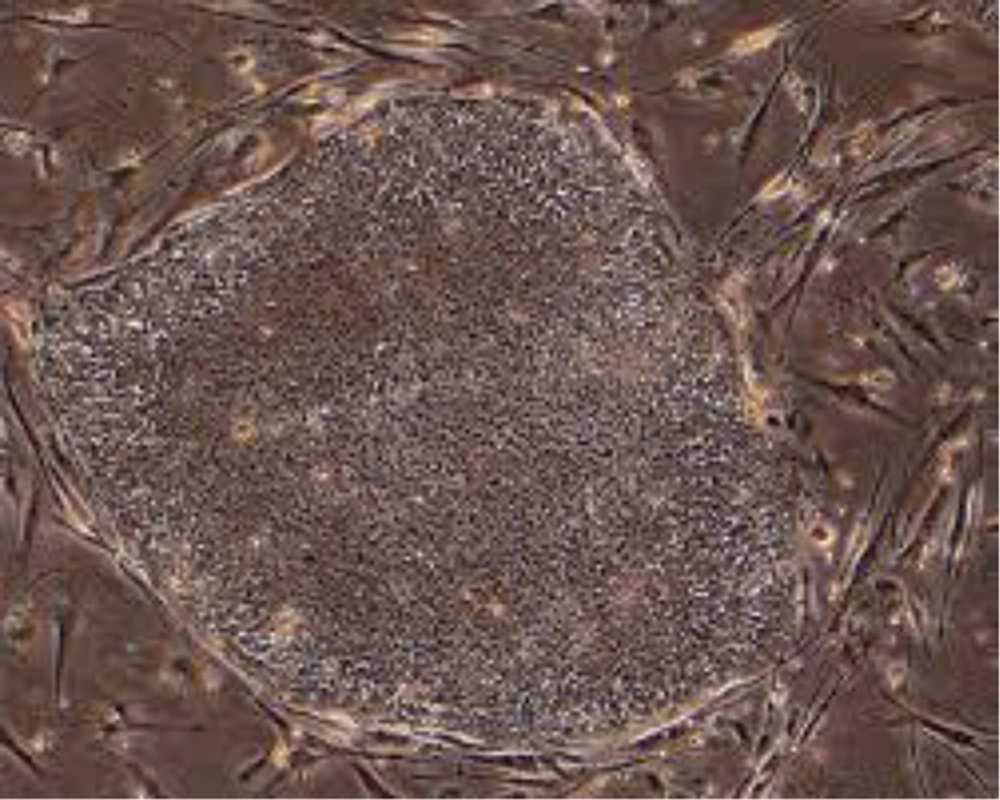
In a study skin fibroblasts reprogramed to induce pluripotent stem cells named iPS cells using viral vector containing four transcription factors Oct-4, Sox-2, c-Myc and Klf4. These cells represent properties the same as ESCs. Skin biopsies from patients are obtained and skin fibroblasts are then reprogrammed into iPSCs. These patient specific-iPSC lines can be differentiated into different cells. A number of diseases have been modeled such as amyotrophic lateral sclerosis, spinal muscular atrophy, Parkinson’s disease, sickle cell anemia and type I diabetes mellitus (29, 30) (Figure 3).