1. Context
Pharmacological therapy has become a major component of antidiabetic regimens. There are numerous cutaneous drug reactions associated with antidiabetic drugs, insulin, and devices. However, the literature is limited, with many reports being case studies and series.
2. Evidence Acquisition
This review provides a brief overview of cutaneous adverse reactions to antidiabetic medications and devices. A PubMed search was conducted using the keywords 'diabetes,' 'cutaneous side effects,' 'insulin,' 'OHA,' 'sulfonylureas,' 'metformin,' and 'antidiabetic drugs.' The resulting articles were analyzed for relevant data and compiled.
3. Results
3.1. Treatment Modalities in Diabetes Mellitus
The following are the available oral antidiabetic medications:
- Dipeptidyl peptidase 4 inhibitors (DPP-4i): DPP-4 inhibitors mimic the actions of incretin mimetics, including stimulation of insulin secretion and inhibition of glucagon secretion.
- Sulfonylureas (glipizide, glyburide, gliclazide, glimepiride): These medications stimulate insulin release from pancreatic β-cells and also improve insulin resistance in peripheral target tissues (muscle, fat).
- Meglitinides: These drugs regulate ATP-dependent potassium channels in pancreatic beta cells and stimulate the release of insulin from pancreatic beta cells.
- Biguanides: Biguanides reduce hepatic glucose output and, to a lesser extent, enhance insulin sensitivity in hepatic and peripheral tissues. They also have an antilipolytic effect that lowers serum free fatty acid concentrations, thereby reducing substrate availability for gluconeogenesis.
- Thiazolidinediones: These medications activate peroxisome proliferator-activated receptor gamma (PPAR-γ), a nuclear receptor, which increases insulin sensitivity and thereby peripheral uptake of glucose. They increase the level of adiponectin, a fat tissue-secreted cytokine, which enhances insulin sensitivity and stimulates fatty acid oxidation.
- α-Glucosidase inhibitors: These inhibitors act on glucosidase and thereby inhibit the breakdown and absorption of carbohydrates (dextrins, maltose, sucrose, and starch; no effect on glucose). They impact postprandial hyperglycemia.
- SGLT2 inhibitors: These medications inhibit sodium-glucose co-transporter 2 (SGLT-2) in the proximal tubules of renal glomeruli, thereby inhibiting glucose reabsorption and causing glycosuria.
- Cycloset (bromocriptine): This is a sympatholytic dopamine D2 receptor agonist. Its action results in the reversal of insulin resistance and a decrease in glucose production. Cycloset, an oral formulation of bromocriptine mesylate, effectively reduces blood sugar by resetting hypothalamic organization of monoamine neuronal activities.
- Glucagon-like peptide 1 (GLP-1) agonists: These medications enhance glucose-dependent insulin secretion, slow gastric emptying, and reduce postprandial glucagon and food intake.
The common types of rashes noted with oral hypoglycemic agents (OHAs) and the commonest agents causing those rashes are depicted in Table 1.
| Type of Reaction | Causative Drugs |
|---|---|
| Photosensitising (a) | Chlorpropamide; Gliquidone; Sitagliptin; Metformin; Canagliflozin; Glipizide; Glyburide; Acetohexamide; Tolazamide; Glipizide; Glimepiride; Glymidine |
| Maculopapular | Metformin; Sulphonylureas; Repaglinide; GLP1 agonists (dulaglutide and liraglutide); SGLT2-I |
| SCAR | Metfomrin; SU; DPP4-I |
| AGEP | SU; |
| Lichenoid reaction | SU; Biguanides |
| Urticaria | Metformin; SGLT2-I; Thiazolidinediones; Insulin |
| FDE | Biguanides; DPP4-I; Dapagliflozin |
3.2. Oral Anti Diabetic Agents
3.2.1. Dipeptidyl Peptidase 4 Inhibitors
DPP-4 inhibitors, also known as ‘gliptins,’ include Sitagliptin, Vildagliptin, Saxagliptin, Linagliptin, Alogliptin, Teneligliptin, Omarigliptin, and Anagliptin. Numerous cutaneous and mucocutaneous adverse events have been reported for DPP-4 inhibitors.
3.2.1.1. Bullous Pemphigoid (BP) and Mucous Membrane Pemphigoid (MMP)
Bullous pemphigoid (BP) is an autoimmune skin disease characterized by tense bullae with linear deposition of IgG and C3 at the subepidermal basement membrane zone, targeting BP antigen 1 and 2 (BP180 and BP230). It comprises 3% of all spontaneous adverse drug reactions to DPP-4 inhibitors (1). The mechanism of drug-induced BP is unclear; however, various theories, such as drug-induced immune dysregulation, molecular mimicry, and drug haptenization of basement membrane proteins, have been proposed.
Among DPP-4 inhibitors, BP is most commonly associated with vildagliptin and sitagliptin, followed by linagliptin and saxagliptin (2). Less than 1% of cases reported are associated with tenegliptin, anagliptin, and alogliptin.
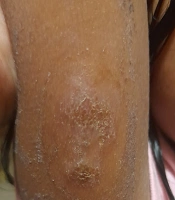
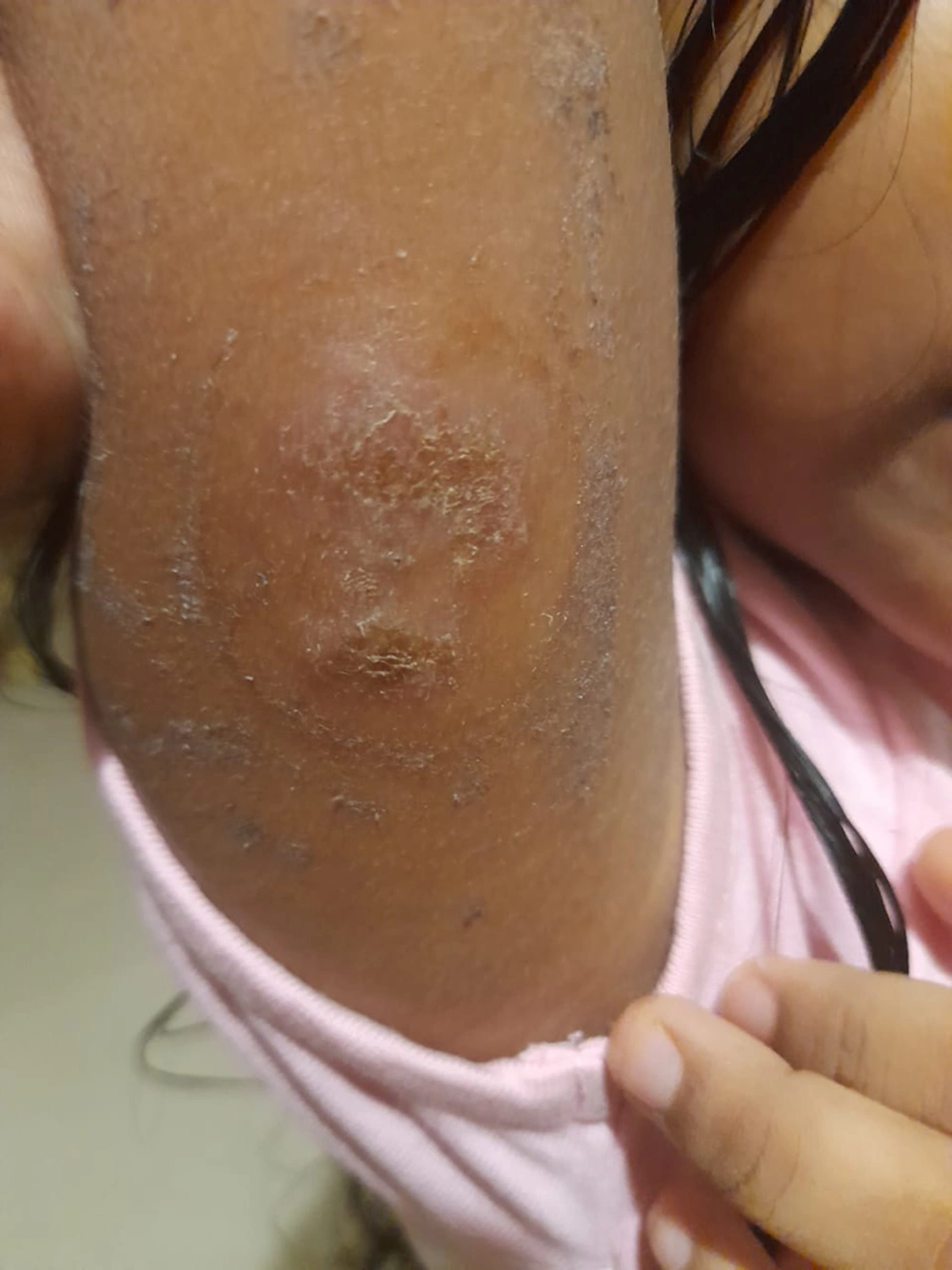
Clinically, the onset of DPP-4 inhibitor-induced BP appears to be at an earlier age than that of spontaneous BP. DPP-4 inhibitor-induced BP manifests either as inflammatory BP (characterized by tense bullae on the trunk, extremities, and face, mostly with an erythematous and edematous base similar to conventional BP) accompanied by intense pruritus, or as a non-inflammatory phenotype (with few, mildly erythematous bullae appearing mostly on normal-appearing skin and with limited distribution). Drug-induced BP can present anytime between 2 to 37 months after medication initiation. (Figure 1)
Gliptin-induced BP has the same histopathological and immunofluorescence (IF) profile as spontaneous BP. Chijiwa et al. reported that in DPP-4 inhibitor-related BP, there is a lesser number of upper dermis eosinophils in the peri-blister lesions compared to patients with spontaneous BP (3). This histological feature was seen in the non-inflammatory phenotype (3).
DPP-4 inhibitors have also been associated with mucous membrane pemphigoid (MMP), involving mucous membranes, commonly of the mouth and eyes. Both BP and MMP are characterized by autoantibodies against BPAG2 but vary in their target domains (NC16A region or C-terminal region, respectively). DPP-4 inhibitor-associated MMP is linked with more cutaneous involvement and less buccal mucosa involvement than non-DPP-4 inhibitor-associated MMP.
3.2.1.2. Angioedema
Angiotensin-converting enzyme (ACE) functions as an enzyme in the breakdown pathway for both bradykinin and substance P, which is why angioedema is quite common with ACE inhibitors. DPP-4 also plays a role in the breakdown of bradykinin and substance P, thereby increasing the risk of ACE inhibitor-associated angioedema when DPP-4 inhibitors are used. The inhibition potency of DPP-4 inhibitors, in descending order, is vildagliptin > sitagliptin > alogliptin > saxagliptin (4).
In a meta-analysis, it was found that when vildagliptin is combined with ACE inhibitors, the risk of angioedema increases (5).
3.2.1.3. Exanthematous Drug Eruption
Teneligliptin has been found to produce idiosyncratic delayed hypersensitivity, manifested as generalized pruritic maculopapular rashes on the trunk and limbs, sparing the mucosa. Agrawal P et al. also reported a similar rash occurring within 2 days after administration of the drug (6).
3.2.1.4. Drug Induced Photosensitive Eruption
Kaori Nakatani reported a sitagliptin-induced drug eruption that occurred 6 months after initiation of the drug. Stopping the drug or protecting from UV light did not completely clear the rash, possibly due to hapten formation with subcutaneous protein and the absorption spectrum of sitagliptin (7). Wavelengths within the UV A (320–400 nm) and UV B (290–320 nm) ranges are more likely to cause drug-induced photosensitivity reactions.
3.2.1.5. Others
It has also been found to cause severe reactions such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and anaphylaxis. Other symptoms include facial edema, hypersensitivity vasculitis, fixed drug eruption, and pruritus. Vildagliptin, when associated with sulfonylureas, has also been noted to cause hyperhidrosis (3).
3.2.2. Sulfonylureas
Cutaneous reactions have been reported with both first and second-generation sulfonylureas. These reactions occur in 2 - 4% of patients taking sulfonamides (8). The mechanism of action is not fully understood. Cutaneous reactions to sulfonamides are often type IV hypersensitivity reactions and include exfoliative dermatitis, exanthematous reactions, psoriasiform rash, exanthematous pustulosis, systemic contact dermatitis, leukocytoclastic vasculitis, erythroderma, Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), pigmented purpuric dermatosis, and erythema multiforme (9-11). The most frequent type is a maculopapular exanthema.
Sulfonylureas, especially chlorpropamide and tolbutamide, may cause contact dermatitis or systemic contact dermatitis in patients who were previously sensitized to certain para-amino-containing compounds. Although rare, cross-reactivity should be expected with para-amino- or sulfa-containing compounds, such as antibiotics. Management involves stopping the offending agent and providing symptomatic treatments, with cutaneous lesions generally resolving within 1–4 weeks (9, 10).
A lichenoid drug eruption has been reported with glimepiride 3 months after starting the drug. Unlike most drug eruptions, where the latent period for the appearance of the eruption is about 1–2 weeks, lichenoid drug eruptions tend to have a longer interval. They clinically appear as a more generalized eczematous or psoriasiform eruption in a photo distribution and do not have Wickham striae, unlike typical lichen planus (12).
Second-generation sulfonylureas are reported less frequently as photosensitizers compared to first-generation sulfonylureas.
3.2.3. Meglitinides
Cutaneous reactions are not very common with meglitinides. The reported types include patch test positive delayed hypersensitivity reactions, maculopapular rashes, and allergic dermatitis (13).
3.2.3.1. -Glucosidase Inhibitors
Though not common, cases of erythema multiforme and acute generalized exanthematous pustulosis have been reported with acarbose (14).
3.2.3.2. Biguanides
Metformin is one of the most commonly used drugs in this category. The most commonly reported reaction to metformin is leukocytoclastic vasculitis (LCV). Other reported cutaneous adverse drug reactions are mostly immunological and include fixed drug eruptions (FDEs), rosacea-like rash, DRESS (Drug Rash with Eosinophilia and Systemic Symptoms) syndrome, psoriasiform, lichenoid drug eruptions, and photosensitivity reactions. A Taiwanese cohort study showed that metformin use was associated with a significantly higher risk of developing chronic urticaria. A longer cumulative duration of metformin use was associated with a higher risk of urticaria (15).
3.2.4. Glucagon‑like Peptide‑1 (GLP‑1) Receptor Agonist
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist that has been found to produce an injection site rash two weeks after subcutaneous administration. The rash resolved upon stopping the drug, leaving deeply seated, hard, firm nodules at the same site (16).
It has also been found to produce exanthematous reactions, such as a morbilliform eruption induced by dulaglutide and a generalized pruritic rash induced by liraglutide. Exenatide has been seen to cause angioedema. This drug has also been implicated in panniculitis, presenting three weeks after starting exenatide, and eosinophilic sclerosing lipogranuloma forming at the injection site (17). Glucagon-like peptide-1 (GLP-1) agonist-associated BP has been reported with dulaglutide and semaglutide.
3.2.5. SGLT 2 Inhibitors
Skin reactions to SGLT-2 inhibitors (SGLT2-Is) are rare but may occasionally be severe enough to warrant discontinuation of the drug. Post-marketing surveillance of SGLT2-Is found 1,136 cases of skin and subcutaneous tissue reactions, with infections being the most common, followed by pruritus, photosensitivity, and urticaria (18).
SGLT-2 inhibitors are associated with a higher risk of genital and urinary tract infections, which often occur earlier than expected when associated with SGLT2-Is. Prior urinary tract infections and obesity are considered predisposing factors. SGLT2 inhibitors have been found to cause candidiasis-induced inflammatory vulvitis with psoriasiform features (19).
Pruritus, along with hyperpigmented and maculopapular rashes, have been reported with empagliflozin and dapagliflozin. There are also reported cases of urticaria, pruritus, photosensitivity, and fixed drug eruption (dapagliflozin). Combining ACE inhibitors and SGLT2-Is leads to an increased ACE2: ACE ratio, which increases the chance of pruritus more than either drug alone (20).
3.2.6. Thiazolidinediones
Adverse skin reactions are less common with thiazolidinediones (TZDs) or 'glitazones.' However, the FDA Adverse Event Reporting System (FAERS) database has recorded cases of urticaria, hyperhidrosis, erythema, alopecia, pruritus, rashes, blistering, hyperkeratosis, palmo-plantar erythrodysaesthesia syndrome, dry skin, and angioedema. Edema has been reported with both rosiglitazone and pioglitazone.
3.3. Insulin
About 3.2 million Indians depend on insulin injections for diabetes management. All newly diagnosed individuals with diabetes who are on insulin should be educated about the correct use of insulin, as incorrect injection techniques may increase the risk of adverse events and poor glycaemic control. New therapies and delivery systems appear to have reduced skin manifestations associated with long-term insulin use. Proper injection technique and the use of shorter needles (4 mm) are associated with improved blood sugar control and lower insulin consumption (21).
The following are the common adverse events noted with insulin injections:
3.3.1. Lipoatrophy
Lipoatrophy refers to the loss of fat in the subcutaneous tissue, presenting as a surface depression. It can occur either because of impurities in insulin preparations or due to the use of non-human insulin. After the introduction of highly purified recombinant human insulin (aspart and lispro) as well as human insulin NPH (neutral protamine Hagedorn), the incidence has noticeably reduced. Lipoatrophy is more commonly reported in Type 1 Diabetes Mellitus (T1DM) than in Type 2 Diabetes Mellitus (T2DM). The development of lipoatrophy takes approximately 4 weeks to 2 years from the time of initiation of insulin analogues (lispro, aspart, glargine, and detemir).
High levels of circulating anti-insulin antibodies with deposition of Immunoglobulin (Ig)M, complement 3 (C3), or fibrinogen at the edge of the lipoatrophic site have been demonstrated in these patients. Lipoatrophy may lead to irregular absorption of insulin.
3.3.2. Lipohypertrophy
Lipohypertrophy is considered to be due to the anabolic action of insulin on fat and can present as a soft swelling. This commonly occurs at sites that are repeatedly injected with insulin. Reduced pain sensation at lipohypertrophic sites often leads patients to inject repeatedly in those areas.
Injection of insulin into sites of lipohypertrophy causes defective absorption and poor glucose control as the tissue becomes fibrous and avascular. Diabetic patients with lipohypertrophy tend to develop hypoglycemic episodes more frequently than those without, and they often require more units of insulin per day.
Risk factors associated with the development of lipohypertrophy include a high total daily dose of insulin, the use of larger needles or reuse of needles, not changing the injection site, HbA1c > 9%, and the duration of diabetes/insulin treatment. Management of lipohypertrophy includes avoiding these risk factors, most importantly by frequently changing injection sites and avoiding reuse of needles.
Lipodystrophies, including both lipoatrophy and lipohypertrophy, can coexist in the same patient.
3.3.3. Insulin Allergy
Allergic reactions to insulin were more commonly observed before the introduction of human insulin and insulin analogues, with a prevalence of about 2%. They were common with bovine and porcine insulin. The reactions could be due to the insulin itself or in response to other components of insulin therapy or impurities in the preparation, including protamine, zinc, and meta-cresol.
Allergic reactions can be either localized or generalized. Local reactions, such as erythema, induration, and papules, manifest at the injection site. Generalized reactions can vary from urticarial rash to an Arthus reaction.
Type I hypersensitivity, mediated by IgE, manifests immediately or within minutes of exposure with localized pruritus, edema, and erythema, or as generalized urticaria, angioedema, and anaphylaxis. A delayed cell-mediated type IV reaction can also be seen 8–12 hours post-insulin administration, peaking at 24 hours, and lasting for several days.
3.3.4. Acanthosis Nigricans
Acanthosis nigricans localized at the site of insulin injection or co-localizing with amyloidosis has been observed with insulin use.
3.3.5. Post Inflammatory Hyperpigmentation
It causes cosmetic disfigurement. Reusing the needle produces more micro-trauma, leading to post-inflammatory hyperpigmentation.
3.3.6. Bleeding and Bruising
Bleeding and bruising can be reduced by using shorter needles. Although bleeding and bruising are quite common with any injectable treatments, they do not affect the absorption or action of injectable therapies.
3.3.7. Amyloidosis
Local amyloid deposition can occur at the site of repeated insulin injections. The nature of the amyloid at the insulin injection site is considered to be insulin itself or an insulin-related substance, known as amyloid insulin type (A Ins). The presence of the amyloid mass may contribute to insulin resistance (22).
3.3.8. Trypanophobia (Belonephobia)
The fear of needles and injections can negatively affect diabetes management.
3.3.9. Others
A case of vitiligo associated with subcutaneous insulin lispro infusion has been published (23).
3.4. Needle Length Recommendations
As injectable therapies play an important role in diabetes management, correct technique is crucial for glycemic control. The Forum for Injection Technique (FIT) India recommendations were developed to address this need (24).
Insulin syringes are available with needle lengths of 6, 8, and 12.7 mm and gauge sizes of 31, 30, and 29. Insulin pens carry insulin in a pre-filled cartridge. The guidelines recommend using needles of 4–6 mm length for both children and adults. However, lean patients should use a skin fold when injecting. Shorter needles have shown equivalent glycemic control, reduced the risk of intramuscular injections, did not increase leakage events, and caused less pain.
Children and adolescents are recommended to use a 4-mm needle with pens and the shortest (4 mm) needles available with syringes. In slim children, a skin fold or angulation of the needle is needed when injecting into the limbs. Absorption is highest over the abdomen, followed by the arms, thighs, and buttocks (25).
3.5. Newer Antidiabetic Devices and Its Cutaneous Effects
The use of devices in diabetes management is becoming increasingly common and includes Continuous Subcutaneous Insulin Infusion (CSII) (insulin pumps) and subcutaneously inserted Continuous Glucose Monitors (CGMs). These devices have become the standard of care for managing type 1 diabetes in pediatric patients. Dermatologic reactions to CSII can occur in response to insulin itself, lack of infusion site maintenance, or reactions to components of the insulin pump.
3.6. Continuous Subcutaneous Insulin Infusion (CSII)/Insulin Pump and Continuous Glucose Monitors (CGMs)
These devices are used for the predictable delivery of insulin to the subcutaneous tissue. The following are the cutaneous adverse reactions noted with their use:
3.6.1. Lipohypertrophy
Among pediatric patients, lipohypertrophy has been reported in 45–47% of cases[26]. Regular monitoring and frequent changes of the insulin infusion sites are important to avoid the development of lipohypertrophy.
3.6.2. Lipoatrophy
In patients with Type 1 Diabetes Mellitus (T1DM) treated with Continuous Subcutaneous Insulin Infusion (CSII), lipohypertrophy accounts for 1% of cutaneous adverse effects (26).
3.6.3. Scars
They comprise 49% of the cutaneous adverse effects associated with CSII use. Repeated needlesticks in the same location with CSII can cause scarring.
3.6.4. Infections
They compose 41% of cutaneous adverse effects related to CSII. These effects can delay insulin absorption. CSII needle colonization is commonly caused by Staphylococcus epidermidis and Staphylococcus aureus. Catheter colonization of devices with bacteria in patients with T1DM has been found to be associated with increased HbA1c, higher body mass index (BMI), and female sex.
3.6.5. Adhesive Components and Contact Dermatitis
In pediatric patients, eczema accounts for 29% and 35% of reported dermatologic complications with CSII and CGM use, respectively (27). Adhesives used in these devices are potential causes of both irritant and allergic contact dermatitis.
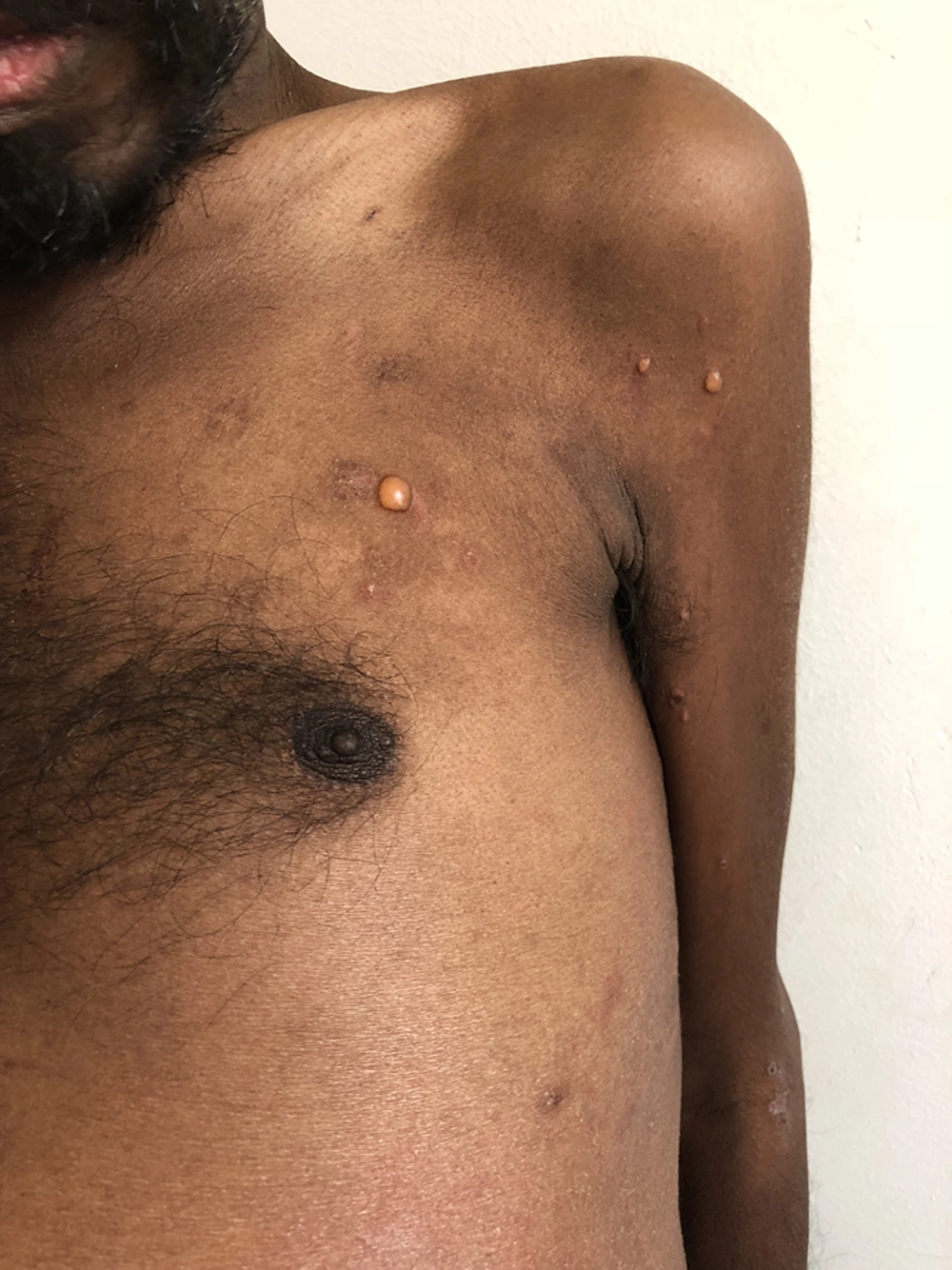
Allergic contact dermatitis has been noted in response to adhesives used in medical devices, including acrylates like ethyl cyanoacrylate, N, N-dimethylacrylamide, colophonium, and epoxy resin; isobornyl acrylate (IBOA) found in plastic materials; and nickel-containing metal injector needles (27). Other reactions to diabetic medical devices include local irritant reactions to plastic tubing containing di-octyl phthalate (Figure 2).
Positive patch test results to other acrylate compounds can occur if a patient is sensitized to one acrylate. Patch testing with 2-hydroxyethyl methacrylate (HEMA) was found to be positive in 80.6% of patients with acrylate allergic contact dermatitis (28).
The common cutaneous side effects of each category are depicted in Table 2. General instructions for proper care of diabetic devices and to reduce adverse reactions are depicted in Box 1.
| Class of Drugs/Devices | Drugs | Common Cutaneous Side Effects | Diagnosis | Management |
|---|---|---|---|---|
| Dipeptidyl peptidase 4 inhibitors | Sitagliptin, vildagliptin, saxagliptin, linagliptin; alogliptin, anagliptin | -Bullous pemphigoid (BP) and mucous membrane pemphigoid (MMP) | Subepidermal blister on histopathology.; linear IgG and C3 deposits on direct immunofluorescence (DIF) on the epidermal side of perilesional skin with salt split technique. | Bullous pemphigoid (BP) and mucous membrane pemphigoid (MMP); stopping the suspected drug.; topical corticosteroid agents; systemic corticosteroids, mycophenolate mofetil, azathioprine, methotrexate, cyclophosphamide, dapsone, doxycycline, and plasmapheresis. |
| Angioedema | Clinical diagnosis | Angioedema; C1esterase-inhibitor; discontinue ACE-inhibitor if present; if no further improvement, discontinue DPP4-i | ||
| Exanthematous drug eruption; photosensitive rash; DRESS syndrome; SJS/TEN; FDE; hypersensitivity vasculitis; | Clinical diagnosis; photopatch test may or maynot be positive; clinical diagnosis; skin biopsy | Exanthematous rash: topical steroids; Discontinue drug; photosensitive rash: Discontinue drug; Photoprotection; DRESS/ SJS/TEN: Discontinue drug; Systemic immunosupressants; Watch for systemic involvement; hypersensitivity vasculitis: Discontinue drug; Systemic steroids | ||
| Sulfonylureas | First generation:Tolbutamide; chlorpropamide; second generation:Glipizide e Glimepiride Glyburide | -Exfoliative dermatitis; exanthematous reactions; psoriasiform rash; exanthematous pustulosis; pigmented purpuric dermatosis; lichenoid drug reactions; systemic contact dermatitis; leukoclastic vasculitis; SJS; erythema multiforme.; photodermatitis | Clinical diagnosis; | Systemic CD: Stop offending agent; symptomatic management; severe adverse drug events/SJS/erythroderma: Stop offending agent; avoid all sulfa compounds; photodermatitis:Withdraw drug; photoprotection topical corticosteroids; symptomatic management; others: Stop offending agent; symptomatic management topical steroids |
| Meglitinides | Repaglinide nateglinide | Maculopapular rashes; allergic dermatitis | Patch test | Withdraw offending drug; symptomatic management |
| Thiazolidinediones/glitazones | rosiglitazone, pioglitazone | Urticaria; hyperhidrosis; pruritis; alopecia; angioedema, hyperkeratosis; palmar-plantar erythrodysaesthesia syndrome; dry skin. | Clinical diagnosis | Withdraw causative drug; symptomatic management |
| Acarbose, miglitol, voglibose | Acute generalized exanthematous pustulosis | Patch test | Withdraw causative drug; Topical steroid | |
| Biguanides | Metformin | Leukocytoclastic vasculitis (LCV); fixed drug eruptions (FDEs); rosacea-like rash, alopecia, DRESS; psoriasiform rash; lichenoid drug eruption; photosensitive reactions; Non vasculitis facial skin eruption; chronic urticaria | Skin biopsy; drug rechallenge test | LCV: Discontinue metformin; oral prednisolone; other immunosupressants;symptomatic management; DRESS: Discontinue causative drug; systemic corticosteroids if there are signs of systemic involvemnet; topical corticosteroids, emollients; others:Stop causative drug; symptomatic management |
| Glucagon‑like peptide‑1 (GLP‑1) receptor agonist: | Exenatide, dulaglutide, liraglutide, semaglutide | Injection site rash/nodule; exanthematous rash; generalized pruritic rash; eosinophilic sclerosing lipogranuloma forming at the injection site; bullous pemphigoid; angioedema; panniculitis | Skin biopsy; Rechallenge | Injection site rash/ Eosinophilic sclerosing lipogranuloma; change injection site frequently; angioedema: Change offending agent; epinephrine oral steroids; antihistamines |
| SGLT2 inhibitors | Empagliflozin,dapagliflozin ; canagliflozin | Pruritus with maculopapular rash; genitourinary infections; urticaria; pruritus; photosensitivity; FDEs | Gram stain, culture and sensitivity and specific diagnostic test for infections | Topical and systemic antibiotics/ antifungals; change offending agent in resistant cases; symptomatic management; good glycemic control |
| Insulin | Animal source: Bovine, porcine; human: Rapid acting and intermediate acting insulin analogues: Ultra short acting: Aspart, lispro; Long acting: Levemir, glargine, Degludec | Lipoatrophy; lipohypertrophy; insulin allergy (localised reactionsat the site of insulin injection to generalized urticarial rashes, angioedema and anaphylaxis); acanthosis nigricans; post inflammatory hyperpigmnetation; bleeding and bruising; amyloidosis; trypanophobia; | Clinical diagnosis; skin biopsy: Lipohypertrophyincrease of groups of local adipocytes separated by fibrous tracts; lipoatrophy: Unilocular adipocytes smaller than that obtained from adjacent normal region. Insulin allergy:Intradermal skin testing; quantification of insulin-specific IgG and IgE in the serum; analysis of the time-dependent binding/dissociation curves of the insulin-neutralizing antibodies in an ex vivo/in vitro assay (29); | Lipoatrophy: Desensitization; change of type of insulin used.; rotating injection sites ; Changes in the delivery system (30);Prophylactic use of topical sodium cromoglycate.(31) subcutaneous injection of insulin and betamethasone into affected areas (28); injected local corticosteroids in combination with insulin; CSII therapy (insulin pump) near the lesion; lipohypertrophy: Change of needles frequently; rotating injection sites; changes in insulin type; insulin allergy: Symptomatic therapy with antihistamines; switch to a different insulin preparation. (32); desensitization/stop insulin/ change the delivery system. Anaphylaxis can be controlled by decreasing the dose or by desensitization (insulin); omalizumab (33). |
| Continuous Subcutaneous Insulin Infusion (CSII)/Insulin Pump and Continuous Glucose Monitors (CGMs) | Lipohypertrophy; lipoatrophy; scars; infections; contact dermatitis | Patch testing for contact dermatitis | Lipoatrophy: Same as above; lipohypertrophy; same as above; infections: Proper skin hygiene; infusion site disinfection; contact dermatitis: Discontinue use of offending product/the device entirely; use of topical corticosteroids; Use of adhesive barriers such as liquid silicone or hydrocolloid plaster or film between the skin and the adhesive part of the sensor. Application of an emollient cream or barrier cream in case of ICD; steroid cream application prior to sensor insertion |
| Variable |
|---|
| Self-inspect sites and avoid areas of infection/inflammation, for device insertion. |
| Teach the patient on correct handing of the device and correct way of insertion. |
| Wash hands thoroughly before inserting cannula |
| Top of insulin vial should be sterilised with alcohol. |
| Infusion sets must be changed every 3 days to prevent it from clogging. |
| Avoid stretching the tape over the skin to make removal easy. |
| Use antimicrobial body washes /antibacterial soaps |
| Perform Glycemic Self-Monitoring: It has a major role in diabetic patients’ self-care. For this procedure, specific lancets for the device should be used and new lancets should be used for each puncture. Hands must be cleansed well before the puncture and lateral aspect of the middle, ring and little fingers should be used for pricking. Patients should be asked to rotate the puncture site. |
3.7. Mimickers of Anti-diabetic Agents Induced Drug Allergy
Hypoglycemia has been found to cause worsening of urticaria (34).
- Non-resolution of urticaria, despite changing OHAs/insulin, should make a clinician suspect that the urticaria is due to poor control of blood sugar levels.
- Bullous diabeticorum: This condition occurs in long-duration diabetes along with other complications. Bullae frequently present bilaterally, involving the lower extremities. Histological findings are often non-specific and show normal immunofluorescence.
- Pruritus in diabetes: Pruritus is the second most common skin manifestation in diabetes mellitus, seen in 15.62% of patients, following infections (35). Poor glycemic control in diabetes mellitus is known to cause pruritus, which can mimic that produced secondary to drugs. Uncontrolled pruritus might at times warrant clinicians to suspect drugs as the cause.
4. Conclusions
The dermatologic adverse effects of antidiabetic medications and medical devices used in the management of diabetes mellitus may vary from mild pruritus to life-threatening conditions. It is essential for dermatologists and practicing physicians to be aware of the common cutaneous side effects caused by these treatments. However, due to limited literature and most cases being reported as individual case reports, a concise review of this topic can aid in the easy analysis and management of these adverse effects, helping to mitigate negative treatment outcomes in patients.