1. Context
Skin maintains homeostasis by temperature regulation via hair follicles, sweat glands and dermal capillaries, and by lubrication via sebaceous glands. The skin is a complex tissue and is the body’s barrier to the outside world (1). Hence, severe skin trauma or burns will lead to a dysfunction of the entire body. The level of skin repair directly affects the living quality of patients. Burn injury represents a cellular stress in the skin. Normal adult skin repair is slow, with high risk of infection and hypertrophic scarring (2). Epidermal keratinocytes form a scar without cutaneous appendages, such as hair follicles, sweat or sebaceous glands. The regenerated epidermis is thin and has fewer and flatter epidermal ridges. Stem cells exist in adult tissue as a reserve to repair cells following stress signals (3).
The vast majority of skin stem cells are located in the hair follicle bulge (4). To repair and heal a wound, stem cells from the hair follicle bulge give rise to daughter skin cells, which migrate to the epidermis (basal layer and sebaceous gland) (5). In adult skin, superficial burns that leave hair follicles intact are healed rapidly with the regeneration of epidermal appendages. Deeper injuries that affect the hair follicle bulge heal with a scar and without adnexal structures (2).
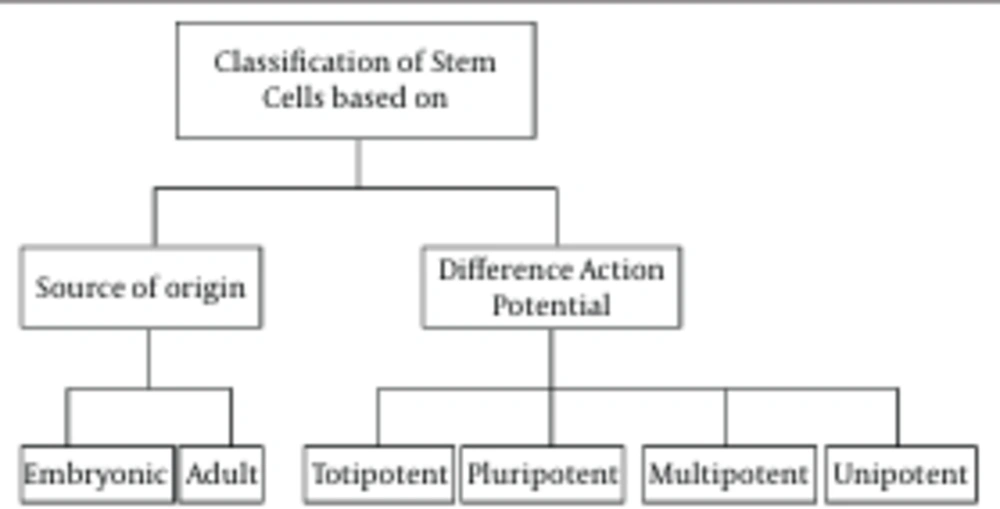
Cutaneous stem cells include epidermal stem cells (interfollicular and bulge stem cells), dermal stem cells, sebaceous stem cells, hair follicle stem cells, sweat gland stem cells, melanocyte stem cells, mesenchymal stem cells, neural stem cells and endothelial stem cells. The most abundant skin stem cells are the epidermal hair bulge stem cells. Only a small fraction of stem cells, the interfollicular stem cells, reside in the basal layer of the interfollicular epidermis. These stem cells maintain adult skin homeostasis and hair regeneration and they also participate in the repair of the epidermis, after trauma.
Besides aiming to accelerate reepithelialization after burn injury, regenerative medicine also focuses on the reconstruction of functional skin with sweat glands, hair follicles and dermal capillaries. These goals might be achieved by stem cell therapy. Approaches to stem cell therapy include local recruitment of endogenous stem cell transplantation (often in vitro modified), either of which can be combined with gene therapy or tissue engineering.
2. Evidence Acquisition
Tissue engineering is an experimental procedure that combines cellular biology, engineering and medicine to develop three-dimensional tissues and restore function (6). Cell therapy has been used to treat burns since the introduction of composite epithelial autografts (CEA) by Green, in 1975, evolving to dermal substitutes, later on to dermal-epidermal bio-engineered cultured skin substitutes and, eventually, to stem cells (7). Stem cell therapy may offer an alternative to large volume resuscitation and be an adjunct to lung-protective ventilation strategies after severe burn injury (8).
Paracrine mechanisms and growth factor secretion, rather than post-engraftment differentiation and proliferation, seem to predominate in the therapeutic effects of multipotent stem cells (MSCs) (7, 9). The MSCs also exhibit anti-apoptotic, immunosuppressive and anti-fibrotic effects (10). For the treatment of acute and chronic non-healing wounds (not burn related), combined gene delivery with stem cell therapy appears promising (11). Gene therapy involves the insertion of a gene into recipient cells by viral transfection, naked DNA application, high pressure injection or liposomal vectors (12). Sequential growth factor gene therapy delivers a cocktail of growth factor genes at strategic time points during wound healing (11, 13).
To enhance the therapeutic response after stem cell treatment in burn patients, intense tissue engineering, with the development of 3D scaffolds or matrices, is of vital importance, as well as improved preconditioning cell treatments and optimized culture conditions (14).
3. Results
We still do not know exactly which percentage of locally delivered cells in a burn wound exerts its effects in wound healing and which, if any, affects the main circulation and has systemic effects, regarding the inflammatory and hypermetabolic response, after a major burn. On the other hand, the efficiency of non-topical administration (such as intrapulmonary infused cells, primarily to treat adult respiratory distress syndrome) for wound healing is unclear. To help clarify this, reporter gene imaging allows for stem cell tracking, in vivo. Currently, three methods, including magnetic resonance imaging (MRI), optical imaging (OI) and positron emission tomography (PET) are used to detect cell markers for the assessment of the viability and location of stem cells, after transplantation (15).
If we focus on wound healing, application of cells to the burn wound could be performed, either by the bedside, as a non-invasive procedure, or in the operating room, immediately after debridement. The cells should be transferred on a matrix, scaffold or dermal substitute. One method is to first spray cells onto the wound with fibrin sealant (16), and, afterwards, cover with a dermal substitute, skin graft or film. Besides acting as a temporary dressing, the cover over the cells acts to theoretically enhance cell paracrine signaling and homing of the cells, improving wound healing (17). Although the use of cell administration using spray technologies is currently being performed in the clinical setting, there are no conclusive data to support its validity, as a matrix vehicle. Cutaneous wound healing requires the well-orchestrated integration of cell migration and proliferation, as well as extracellular matrix deposition, angiogenesis, and remodeling (11).
4. Conclusions
Delays in burn wound closure worsen a patient’s susceptibility to infection, prolong pain, increase the total number of operative procedures, increase the incidence of hypertrophic scarring, and lengthen hospital stays. Stem cell therapies in wound care may lessen these morbidities (18). Specifically, the burn wound has unique characteristics that have to be considered when designing a clinical trial for stem cell therapy applications: it is an ischemic wound, with an altered pH and temperature, prone to infection and development of chronic sequel, such as non-healing ulcers and hypertrophic scarring (19, 20). Furthermore, a major burn represents a handicap, with uncovered wounds open to air, which requires frequent operations and dressing changes, and with long periods of immobilized hospital stay, which involve frequent position changes and physiotherapy, to avoid pressure sores, enhance rehabilitation and improve overall prognosis. This dynamic paradigm popularized the use of polymeric films for the repair and closure of wounds. These films are semipermeable and transparent materials that create an accelerated healing environment while avoiding dehydration, trauma and infection over the injury (21). Moreover, radiofrequency applied to wound-contacting iron oxide nanoparticles has been used to debride the wound may represent a novel burn treatment method, once stronger scientific evidence is available (22).
An ideal method for the effective administration of stem cells for burn patients has not yet been elucidated. Further comparison of the local and systemic effects in burn patients, associated with each route of stem cell delivery, needs to be performed. There is still not enough evidence in terms of analyzing systemic or local effects of stem cell delivery in burn patients, regarding different possible routes of administration.