1. Context
1.1. Cell Therapy
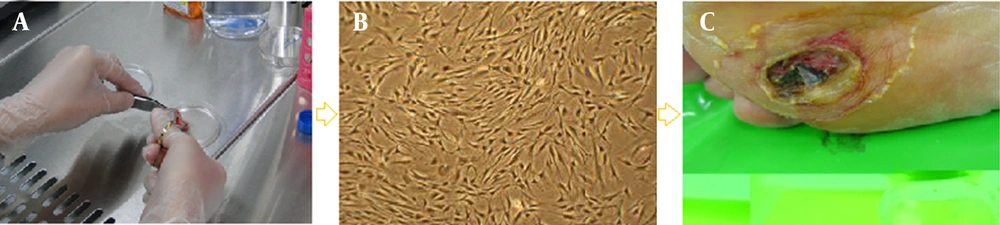
Cell therapy, first introduced in the nineteenth century, is defined as the transplantation of cellular material into a patient. Various cell therapies are emerging worldwide as new treatment modalities in clinical medicine. In this process, autologous or allogeneic cells and materials are propagated, expanded, selected, pharmacologically treated, or otherwise altered in biological materials to prevent, treat, or diagnose disease. For example, standard approaches to improve wound healing may speed re-epithelialization, but skin function will remain compromised; a novel approach to promote skin regeneration is the introduction of fibroblasts into the wound (Figure 1) (1).
1.2. Recessive Dystrophic Epidermolysis Bullosa (RDBE)
RDBE is a group of autosomal-dominant blistering skin diseases caused by mutations in the collagen VII gene (COL7A1) and subsequent abnormalities in the anchoring fibrils (collagen type VII) (2). Collagen VII is the major constituent of the basement membrane, and defects in it are characterized by dermal-epidermal separation below the lamina densa (3). It has been demonstrated that COL7A1 is not only vital for maintaining skin integrity, but is also a critical player in skin-wound healing (4).
The DDEB phenotype is characterized by recurrent blistering; milia; atrophic scarring; erosions; crusts; nail dystrophy with eventual loss of nails; functionally disabling contractures in the hands, feet, elbows, and knees; and a high risk of developing aggressive squamous cell carcinoma (5).
Skin consists of two layers, the epidermis and the dermis, the main cells of which are keratinocytes and fibroblasts, respectively. In vivo, both fibroblasts and keratinocytes produce collagen VII (6); however, keratinocytes are the major cell source for its synthesis in the basement membrane zone (7). It is easier to culture fibroblasts than keratinocytes, and therefore fibroblasts may be a superior cell-source target for skin-cell therapy (8).
2. Evidence Acquisition
2.1. Fibroblast Features
Fibroblasts, the most common cells in connective tissues, are resident mesenchymal cells that produce extracellular matrix (ECM) proteins. Fibroblasts are major cellular constituents of the skin dermal layer, expressing the intermediate filament protein known as vimentin, which is used as a marker to test their mesodermal origin. However, this test is not specific, as epithelial cells cultured in vitro on adherent substratum may also express vimentin (9).
2.1.1. Types of Fibroblasts
Fibroblasts in different tissues have different morphologies and gene-expression profiles. ECM proteins and cytokines are synthesized by fibroblasts in a tissue-specific manner (10, 11). Dermal fibroblasts have numerous functions, not only in synthesizing ECM proteins, but also in proliferation and migration in response to chemotactic, mitogenic, and modulatory cytokines, and in autocrine and paracrine interactions (12). Dermal fibroblasts have been evaluated for other applications, such as ligament regeneration (13).
2.1.2. Fibroblast Secretion Materials
In the dermis, fibroblasts produce different types of collagens (I, III, and VII), elastic fibers (elastic, oxytalan), matrix metalloproteinases (MMPs), glycosaminoglycans, glycoproteins, and cytokine human thymic stromal lymphopoietin (TSLP) (Box 1) (14).
| Materials |
|---|
| Collagens (type I, III, and VII) |
| Elastin |
| Hyaluronic acid (hyaluronan) |
| Matrix metalloproteinases |
| Glycosaminoglycans |
| Reticular and elastic fibers |
| Cytokine human thymic stromal lymphopoietin |
3. Results
3.1. Wound Healing and Fibroblasts
Fibroblasts are known to play a pivotal role in skin structure and integrity, and dermal fibroblasts are believed to promote skin regeneration and rejuvenation via collagen production (15).
Wound healing is a complex process that involves four phases: hemostasis, inflammation, granulation (proliferation), and maturation (remodeling). In hemostasis, platelets seal off the damaged blood vessels within minutes of the initial injury to reduce blood loss, and fill the tissue gap with a blood clot. In the inflammation stage, plasma infiltrates through the blood vessels, and neutrophils immigrate into the surrounding tissue, where they phagocytose the debris and microorganisms for four days post-injury. During the inflammation phase, circulating monocytes exit the blood vessels, differentiate into macrophages, and come into contact with the ECM. Macrophages also phagocytose bacteria and provide a second line of defense. Under the influence of inflammatory cytokines, macrophages secrete extracellular enzymes to degrade the necrotic tissue. These enzymes, called matrix metalloproteases (MMPs), are responsible for removing the necrotic tissue and repairing the remaining damaged tissue.
Approximately four days after the injury, the proliferation phase occurs. This is characterized by angiogenesis, fibroblast proliferation and differentiation, collagen deposition, granulation tissue formation, wound contraction, and epithelialization. During this phase, fibroblasts secrete the collagen framework, on which further dermal regeneration occurs. During the proliferation process, ECM proteins act as scaffolds for migrating inflammatory cells and the generation of granulation tissue, which provides the temporary substrate upon which re-epithelialization by keratinocyte cells takes place (16). Fibroblasts also have the capacity to differentiate into myofibroblasts, which generate wound contracture. The keratinocytes are responsible for re-epithelialization in the final stage of epithelialization (17). In response to the wound, macrophages and fibroblasts also release growth factors that lead to further fibroblast migration and proliferation (18).
In the remodeling phase of wound healing, myofibroblasts undergo apoptosis, resulting in decreased cell density, while the remaining dermal fibroblasts begin to produce type I collagen in order to create greater tensile strength. This plasticity of fibroblasts as they transition from producing ECM to promoting wound contracture, and their synthesis of type 1 collagen, makes them a favorable candidate for cellular therapy in wound healing (11).
3.1.1. Application of Fibroblasts in Skin Cell Therapy
Dermal fibroblasts are used in the treatment of surgical and burn wounds, chronic wounds such as diabetic and pressure ulcers, and acne scars, as well as for cosmetic purposes, such as treating facial rhytides and skin-aging (17).
3.2. Allogeneic Fibroblasts
The fibroblasts used in skin cell therapies may be allogeneic or autologous. Autologous fibroblasts carry no risk of rejection or cross-infection, but autologous fibroblasts require 3 - 4 weeks of cultivation to obtain sufficient cell numbers; therefore, allogeneic fibroblasts are a superior source for cell therapies (19), and they are often obtained from foreskin samples. After isolation, these cells are cultured, then cryopreserved at −196°C (20).
3.2.1. Immunogenicity of Allogeneic Fibroblasts
The viability of allogeneic fibroblasts implanted into a wound remains controversial because of possible immune rejection. However, there have been 88000 fibroblast cultures implanted into wounds, corresponding to more than 20000 patients, without any observed acute immune rejections. The absence of antibody formation against allogeneic fibroblasts is seen in 50% of patients, and the absence of T-cell activation is reported in 25% of patients (13).
It has been reported that after allogeneic fibroblast transplantation, these cells should be able to integrate with the host tissues (20). Allogeneic living fibroblasts are known to have been successfully applied in different types of wounds (21).
Autologous dermal fibroblast transplantation holds enormous promise in the field of regenerative medicine, offering a simple alternative for tissue-regeneration applications. Basic research into the mechanism(s) of regional phenotype determination by fibroblasts will help in the development of reprogramming options for cell therapy applications (22).
3.3. Therapeutic Indications
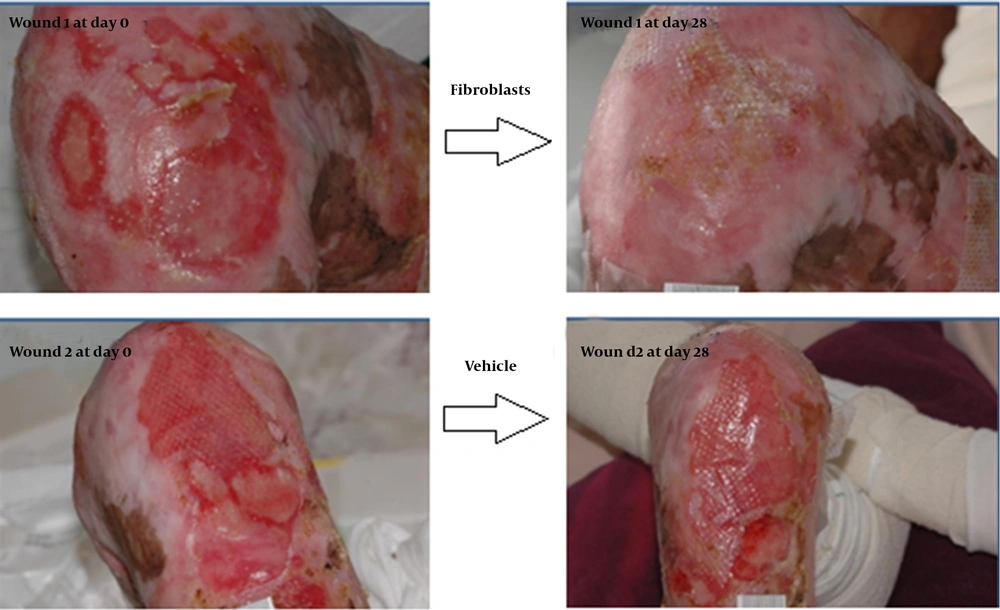
In a double-blinded, randomized, placebo-controlled trial that assessed allogeneic fibroblasts in RDEB, 20 patients were screened; seven were excluded and five were selected. Six pairs of symmetrical lesions in each patient were selected, and one side received a single intradermal injection of cultured allogeneic fibroblasts while the other side received vehicle solution (Transalyte with 2% Albumex). After 12 months of follow-up, skin biopsy was performed, and blood tests, quality of life questionnaires, and wound dimensions were assessed. No benefit was detected with allogeneic fibroblasts, as similar improvement was described in both groups.
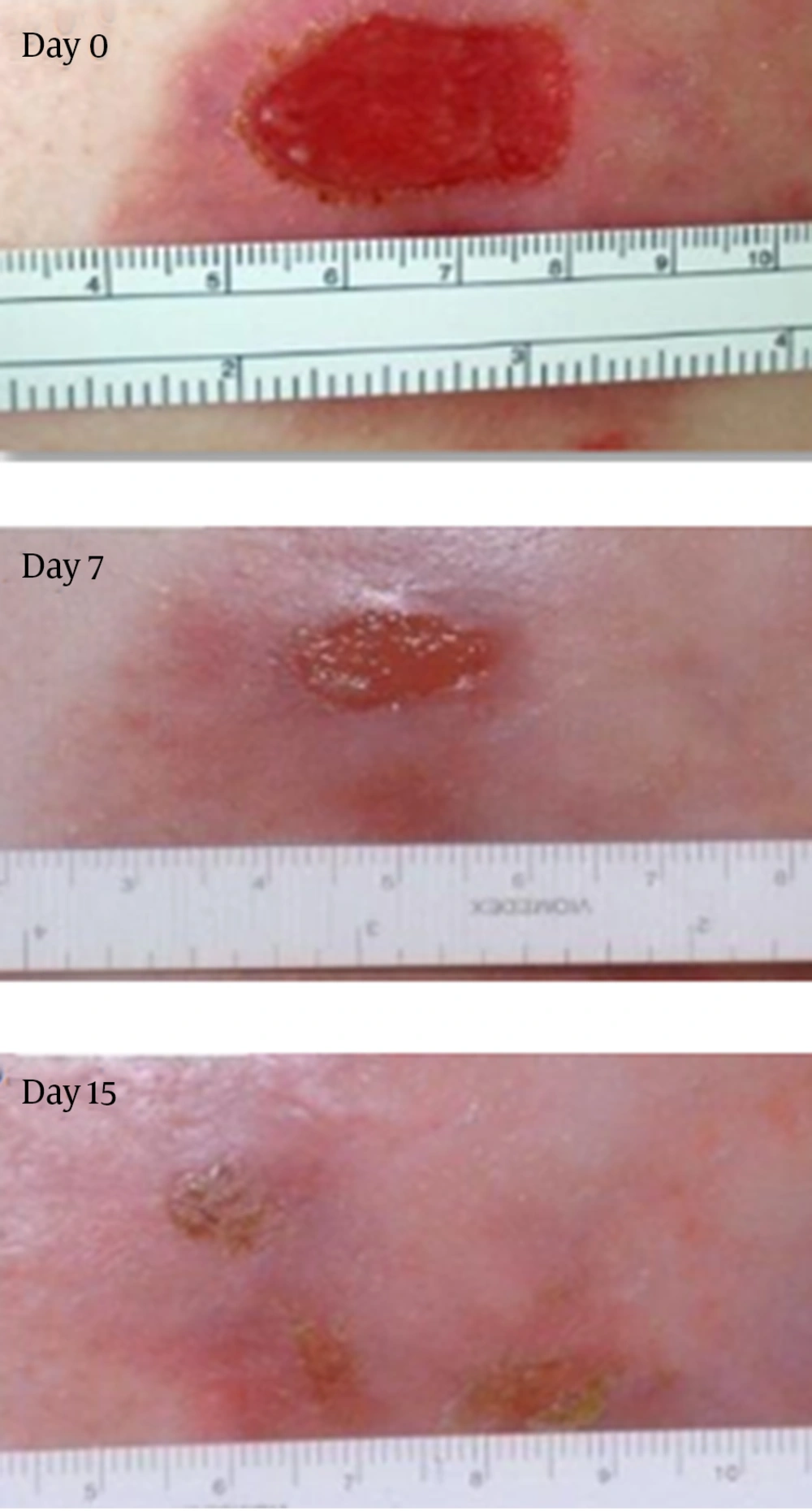
In an open-label study, injecting cultured allogeneic fibroblasts into RDEB wounds led to clinical improvement in some patients. A recently completed Phase II clinical trial of fibroblast cellular therapy for RDEB built on a great deal of laboratory research and the previous pilot trial, in which 15 patients with RDEB showed that injections of fibroblasts from unrelated donors into the skin were apparently safe and had the potential to improve skin strength and to reduce blistering. The majority of the wound healing benefits developed during the first 28 days of a single injection (23) (Figures 2 and 3).
3.4. Bio-Engineered Skin
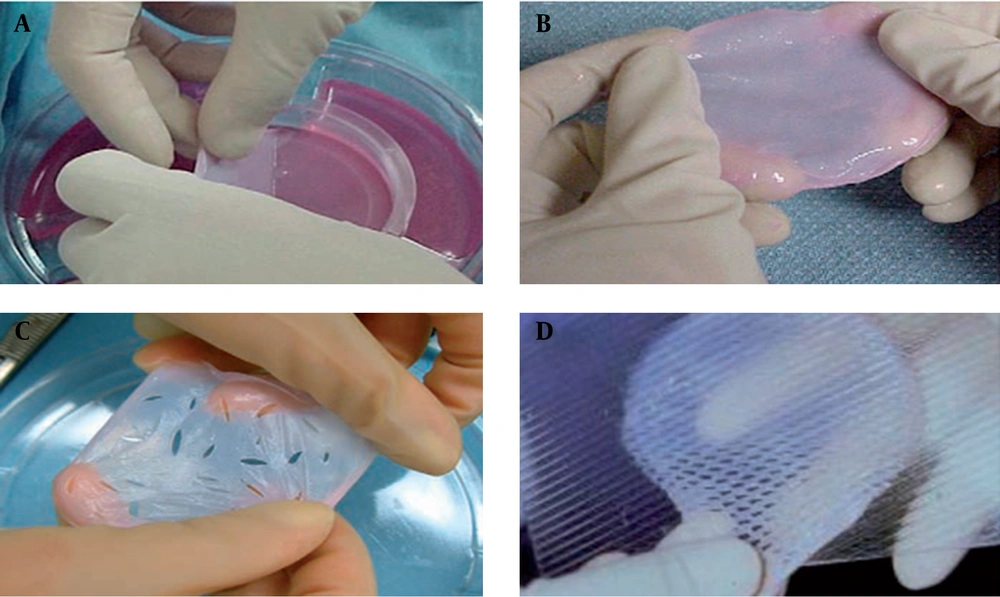
Artificial skins and soft-tissue substitutes may be either acellular or cellular. Acellular products contain a matrix composed of materials such as collagen and fibronectin (24). Cellular products contain living cells, including fibroblasts and keratinocytes, within a matrix. Such products are widely used in clinics and include Dermagraft, Hyalograft, Orcel, Apligraf, ICX-SKN, and VCT01 (Box 1 and Table 1) (Figure 4) (25).
| Manufacturer | Brand Name | Material |
|---|---|---|
| Organogenesis, Canton, MA | Apligraf™ (earlier name: Graftskin™) | Collagen gel + cult. Allog. HuK + allog. HuFi |
| Genzyme Biosurgery, Cambridge, MA | Epicell™ | Cult. Autol HuK |
| Advanced Tissue, La Jolla, CA | Transcyte™ | PGA/PLA + ECMP DAHF |
| Integra LifeScience, Plainsborough, NJ | Integra™ | Collagen GAG-silicone foil |
| Lifecell Corporation, Branchberg, NJ | AlloDerm™ | Acellular dermis |
| Fidia Advanced Biopolymers, Padua, Italy | Laserskin™ | HAM + cult. HuK |
| Advanced Tissue Sciences, La Jolla, CA | Dermagraft™ | PGA/PLA + allog. HuFi |
| Ortec International, Inc., New York, NY | Orcel™ | Collagen + allog HuFi + allog HuK |
| BioTissue Technologies, Freiburg, Germany | Bioseed™ | Fibrin sealant + cult. autol HuK |
| HC Implants | Polyactive™ | PEO/PBT + autol. HuFi + cult autol HuK |
| Fidia Advanced Biopolymers, Padua, Italy | Hyalograft 3D™ | HAM + HuFi |
| Dow Hickham/Bertek Pharmac., Sugarland, TX | Biobrane™ | Silicone + nylon mesh + collagen |
4. Conclusions
Fibroblasts can be used in transplantations to ameliorate an immune system response, in order to reduce antigen production. Human fibroblasts suppress ongoing mixed lymphocyte reactions (MLRs) between lymphocyte cells from two individuals, and supernatant materials from fibroblast cultures suppress MLRs. Supernatants from fibroblast cultures of different individuals do not exhibit specificity of the reduced response with regard to major histocompatibility complex type. The collected data indicate that allogeneic fibroblasts are capable of surviving at a wound site for at least six months, and we did not have any findings of acute immunologic rejection of allogeneic fibroblasts. Further studies are needed to evaluate the mechanism of action of dermal fibroblasts in promoting the formation of new type VII collagen in wounds.