1. Background
Autologous fat grafting is widely used to address reconstructive and aesthetic issues in both adult and pediatric populations (1). A wide range of conditions, including congenital anomalies like hemifacial atrophy, pectus excavatum, cleft lip, subcutaneous soft tissue defects post-resection, and post-traumatic defects of the face and body such as burn injuries or postoperative scars, are treated with adipose tissue grafts (1). While autologous fat grafts are extensively utilized in adults, their application in pediatric patients is limited, primarily due to children’s relatively restricted fat tissue reserves. Extensive research on the use of autologous fat grafting in children and adolescents remains crucial to fully understanding its potential benefits in pediatric healthcare.
Adipose tissue, a rich source of mesenchymal stem cells, primarily consists of mature adipocytes, extracellular matrix, and stromal vascular fraction, which contains adipose tissue-derived stem cells, preadipocytes, fibroblasts, smooth muscle cells, and immune cells (2). Preadipocytes are the precursors of mature adipocyte cells. Unlike mature fat cells, preadipocytes differentiated from mesenchymal stem cells within the stromal-vascular section of adipose tissue retain the potential to proliferate and differentiate throughout their lifespan (3). With these characteristics, preadipocytes present a promising cell source for soft tissue augmentation.
2. Objectives
This study aims to compare the volumetric and histopathologic changes of preadipocyte fat grafting with conventional adipose grafting, highlighting the feasibility of autologous preadipocyte tissue transplantation, even in cases with limited adipose tissue supply.
3. Methods
3.1. Experimental Design
A total of 28 male Wistar albino rats weighing 250 - 300 g were included in the study. All procedures were carried out according to the guidelines for the care and use of laboratory animals at Adnan Menderes University. The animals were housed in standard cages, subjected to a 12-hour light/12-hour dark cycle, provided ad libitum access to water and rat chow, and monitored closely throughout the study period. The rats were divided into four groups according to the timing of excision: Group 1 (n = 7) at 3 weeks post-grafting, group 2 (n = 7) at 6 weeks post-grafting, group 3 (n = 7) at 10 weeks post-grafting, and group 4 (n = 7) at 22 weeks post-grafting.
3.2. Adipose Tissue Harvesting
For the procedure, all rats were anesthetized with an intramuscular injection of 5 mg/kg xylazine and 50 mg/kg ketamine. Following abdominal shaving and application of a 5% iodine solution, a 2 g fat sample was harvested from the right epididymal fat pad through an abdominal incision in each rat. The adipose tissue samples were promptly transported to the laboratory in sterile plastic containers containing basal medium.
3.3. Preparation of the Preadipocytes Suspensions
The isolation and primary culture of preadipocytes were conducted following the method proposed by van Harmelen et al. (4). Fat tissue samples were placed in Petri dishes containing phosphate-buffered balanced salt solution (PBS) within a laminar flow cabinet and finely dissected with scissors. Visible connective tissue, debris, and blood vessels were removed. The dissected adipose tissue was transferred to tubes, and collagenase solution (3 mL solution per 1 mL fat tissue) was added. The sealed tubes were placed horizontally in a shaker bath at 37°C for approximately 90 minutes to facilitate digestion. After digestion, the tubes were centrifuged at 200 g for 10 minutes at room temperature. The supernatant, containing mature adipocytes and collagenase solution, was separated from the pellet containing the preadipocytes. A 1 mL solution was left at the bottom to prevent the pellet from drying out. To remove erythrocytes, an erythrocyte lysis buffer [155 mmol NH4Cl, 5.7 mmol K2HPO4, 0.1 mmol ethylenediaminetetraacetic acid (EDTA)] was added to the tube and incubated. The suspension was then filtered through a 150-μm filter and re-centrifuged. The supernatant was discarded, and 10 - 20 mL of basal medium, consisting of an equal volume of Dulbecco's Modified Eagle Medium (DMEM) and F12 solution, plus 15 mmol 4-(2-hydroxy-ethyl)-1-piperazine ethane sulfonic acid (HEPES), 14 mmol NaHCO3, 33 μM biotin, and 17 mmol D-pantothenate, was added to the pellet. After filtering through a 70-μm filter, cell counts were determined using a Neubauer hemocytometer, and cell viability was assessed with trypan blue staining.
An inoculation medium of 1 million cells/mL was prepared by adding gentamicin (50 mg/mL) and 10% fetal bovine serum (FBS) to the basal medium. The cells were transferred to culture plates at an inoculation density of 30,000 - 50,000 cells/cm² and incubated in the inoculation medium at 37°C and 5% CO₂ for 16 - 24 hours for optimal cell adhesion. Once cells adhered, they were washed twice with warm PBS to eliminate non-adherent cells. A preadipocyte basal culture medium was prepared by adding insulin (66 nmol), triiodo-L-tyrosine (1 nmol), transferrin (10 mg/mL), and gentamicin (50 mg/mL) to the basal medium to promote preadipocyte production. The cells in the culture plates were observed daily under an inverted microscope. Spindle-shaped preadipocytes reached confluence in the culture plate within 7 - 9 days, at which point the cells were detached from the plates using trypsin/EDTA solution. The cells were recounted, and the solution was diluted with PBS to a cell density of 10,000 - 30,000 cells/mL. Cell suspensions were then incubated with 5 mL of immunofluorescent dye PKH26 (Sigma, St. Louis, MO, USA) for five minutes. Residual dye was removed by washing the cells three times with PBS solution.
3.4. Preparation of the Adipocyte Suspensions
The isolation and primary culture of preadipocytes were conducted following the method proposed by van Harmelen et al. (4). Fat tissue samples were placed in Petri dishes containing PBS within a laminar flow cabinet and finely dissected with scissors. Visible connective tissue, debris, and blood vessels were removed. The adipose tissue fragments were transferred to tubes, and collagenase solution was added. The sealed tubes were placed horizontally in a shaker bath at 37°C for approximately 90 minutes to facilitate digestion. At the end of this process, a homogeneous adipocyte solution was obtained. The cells were counted using a Neubauer hemocytometer and diluted with PBS to achieve a cell density of 10,000 - 30,000 cells/mL. The cell suspension was incubated with 5 mL of immunofluorescent dye PKH26 for five minutes. Residual dye was removed by washing the cells three times with PBS solution.
3.5. Graft Implantation
After shaving the scapular region and marking each injection site, a 1 mL preadipocyte suspension was introduced subcutaneously on the right side of the scapular area using a 21-gauge needle attached to a 10 mL syringe. A vacuum effect was applied to the tissue by pulling the syringe plunger upwards, and 1 mL of the preadipocyte suspension was slowly injected into the right scapular area. The vacuum application was maintained for 3 minutes to prevent the spread of the cell suspensions from the subcutaneous tissue. Using the same method, 1 mL of adipocyte suspension was injected into the left scapular area with an 18-gauge needle. The injection sites on the right and left scapular areas were excised at 3, 6, 10, and 22 weeks, according to each group's timeline.
3.6. Evaluation
3.6.1. Volumetric Evaluation
Right and left scapular fat tissue corresponding to the injection sites was excised under general anesthesia, and the width, length, and height were measured. The hemi-ellipse formula [(2/3π) × (a) × (b) × (c)] was used to evaluate the volume of the excised tissue, where a, b, and c represent the width, length, and height radius of the excised tissue, respectively. The volumes of the excised fat tissues, where preadipocyte cell suspensions and adipocyte cell suspensions were grafted, were calculated in each group and compared.
3.6.2. Histologic Evaluation
Tissue samples were divided into two portions. One portion was frozen at -40˚C, and 4 µm thick sections were prepared for assessment with the immunofluorescent dye PKH26 (Sigma, St. Louis, MO, USA). Stained specimens were evaluated under fluorescence microscopy.
The remaining tissue samples were immersed in 10% neutral buffered formalin, subsequently embedded in paraffin, and sectioned into 5 µm slices. Slides were processed for histological staining with hematoxylin and eosin. After staining, an experienced histologist, blinded to the experimental conditions, evaluated the slides using an Olympus IX71 microscope (Olympus, Center Valley, PA). The samples were assessed for indicators such as necrosis, fibrosis, vascularity changes, cyst formation, and inflammatory cell density, with scores ranging from 0 to 2 assigned for each parameter, as outlined in Table 1. Total histopathology scores were calculated for both preadipocyte and adipocyte grafts within each experimental group.
| Criteria | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Necrosis | None | Mild | Severe |
| Fibrosis | None | Mild | Severe |
| Vascularity | Increased | Normal | Mild |
| Cyst formation | None | Visible | - |
| Inflammatory cell density | Mild | Normal | Increased |
3.7. Statistical Analysis
All results were expressed as mean ± SD and median (minimum-maximum). Statistical analysis was performed using GraphPad Instat 3.0 (San Diego, CA, USA) and SPSS 17.0 statistical software. One-way analysis of variance (ANOVA) and Tukey's multiple comparison post hoc test were used for intra-group comparisons, while Wilcoxon's rank-sum test was employed for comparing adipocyte and preadipocyte groups across the different weeks. Significance was accepted at P < 0.05.
4. Results
4.1. Assessment of Graft Volume Retention
Animals were sacrificed at designated weeks according to their groups, and grafts were dissected. When the volumes of preadipocyte and adipocyte grafts were compared, a noticeable decline in volume was observed over time in both tissue types. This volumetric reduction was more pronounced in adipocyte grafts; however, despite these differences, they were not statistically significant in group 1 (3 weeks), group 2 (6 weeks), and group 3 (10 weeks). In group 4 (22 weeks), however, preadipocytes retained their size, and the volumetric loss in adipocyte grafts was significantly more substantial compared to the preadipocyte grafts (0.09 ± 0.01 vs. 0.05 ± 0.02, P < 0.05). A comprehensive overview of graft volumes for both preadipocytes and adipocytes is provided in Table 2.
| Groups and Categories | Mean ± SD | Median | Minimum-Maximum |
|---|---|---|---|
| I | |||
| Preadipocyte | 0.13 ± 0.05 | 0.12 | 0.06 - 0.21 |
| Adipocyte | 0.12 ± 0.06 | 0.12 | 0.01 - 0.21 |
| II | |||
| Preadipocyte | 0.12 ± 0.06 | 0.13 | 0.06 - 0.21 |
| Adipocyte | 0.11 ± 0.06 | 0.11 | 0.03 - 0.21 |
| III | |||
| Preadipocyte | 0.09 ± 0.05 | 0.08 | 0.03 - 0.20 |
| Adipocyte | 0.08 ± 0.02 | 0.09 | 0.03 - 0.10 |
| IV | |||
| Preadipocyte | 0.09 ± 0.01 b | 0.10 | 0.07 - 0.12 |
| Adipocyte | 0.05 ± 0.02 b | 0.05 | 0.03 - 0.09 |
a Values are expressed as number (cm³).
b P < 0.05.
4.2. Histopathological Results
Immunologic evaluation using PKH26 (Sigma, St. Louis, MO, USA) confirmed that the grafted preadipocytes and adipocytes remained viable and well-preserved at the recipient site. Histopathological examination provided crucial insights into the immunologic response of preadipocytes and adipocytes following grafting, revealing that preadipocytes exhibited significantly lower total histological scores compared to adipocytes across all experimental groups (Table 3). These findings indicate a notable discrepancy in graft outcomes between the two cell types (Figure 1).
| Groups and Categories | Mean ± SD | Median | Minimum-Maximum |
|---|---|---|---|
| I | |||
| Preadipocyte | 4.14 ± 1.46 | 4.0 | 2.0 - 6.0 |
| Adipocyte | 7.00 ± 2.16 | 8.0 | 3.0 - 9.0 |
| II | |||
| Preadipocyte | 3.28 ± 0.48 | 3.0 | 3.0 - 4.0 |
| Adipocyte | 5.75 ± 1.58 | 6.0 | 3.0 - 8.0 |
| III | |||
| Preadipocyte | 2.75 ± 0.89 | 2.5 | 2.0 - 4.0 |
| Adipocyte | 5.42 ± 1.39 | 6.0 | 3.0 - 7.0 |
| IV | |||
| Preadipocyte | 2.85 ± 0.76 | 3.0 | 2.0 - 4.0 |
| Adipocyte | 4.71 ± 1.53 | 4.0 | 3.0 - 8.0 |
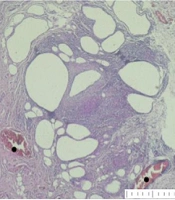
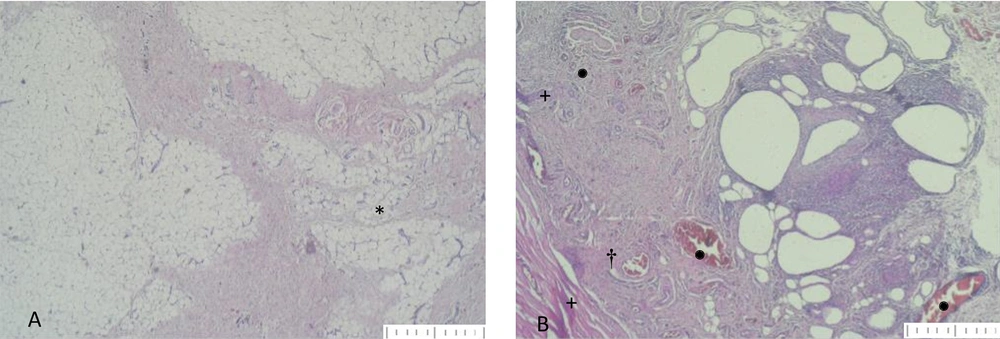
A, *: Fat tissue structure formation from the preadipocyte grafts in group 4 (H&E stain, × 40 magnification); B, cyst formation from adipose grafts from group 4. †: Infiltration of inflammatory cells around the cystic areas; +: Necrosis formation; •: Vascular structures. (H&E stain, × 40 magnification)
5. Discussion
The term "soft tissue defect" refers to a deficiency in connective tissue at any level, often leading to disruption of the natural tissue contour (5). Despite advancements in medical knowledge and technological innovations in correcting soft tissue defects, these remain challenging for surgeons, with complexity heightened in pediatric patients. The limited amount of adipose tissue, combined with concerns about altering the contour of the donor site to achieve better cosmesis elsewhere, makes autologous adipose grafting a significant dilemma, especially in the pediatric population.
Adipose tissue-derived stem cells have gained widespread use in cellular therapy due to their remarkable ability to differentiate into diverse cell types and their significant anti-inflammatory, anti-fibrotic, and pro-angiogenic effects (6). Both in vivo and in vitro studies have demonstrated preadipocytes’ capacity to differentiate into mature adipose cells (7). In contrast to the limited regenerative capabilities of adipocytes, preadipocytes offer greater potential for soft tissue augmentation due to their ability to differentiate and proliferate throughout their entire lifespan (8).
The ultimate success of fat grafting depends on efficient revascularization and histological harmony between donor and recipient sites. Post-graft inflammation, fibrosis, and cyst formation are extensively studied in the literature. Trotzier et al. assert that the inflammatory response at the donor site reduces angiogenesis and blood flow to the graft, ultimately diminishing graft survival (9). Conversely, preadipocytes do not typically elicit a host reaction or provoke an inflammatory response, focal cell necrosis, cyst formation, or fibrosis in experimental studies (10, 11). Bellini et al. attribute preadipocytes' resistance to hypoxia to the inherent characteristics of progenitor cells (12). Their minimal metabolic activity and lower oxygen consumption rates enable them to endure extended periods without nutritional supply.
In this study, histopathologic assessment was conducted using a total scoring system consisting of necrosis, fibrosis, cyst formation, vascularity, and inflammatory cell density. Notably, the scores for preadipocyte groups were significantly better than those for adipocyte groups across all cohorts (P < 0.05). PKH26 evaluation played a pivotal role in reaffirming the viability and robust protection of grafted preadipocytes and adipocytes within the recipient site. Our comprehensive histopathological examination provided valuable insights into the subsequent immunologic responses of these cell types following grafting. It was consistently observed that preadipocytes exhibited significantly lower total histological scores than adipocytes across all experimental groups. This significant difference highlights a substantial variance in graft outcomes between these two cell populations, likely influenced by variations in immune system interactions, cellular behaviors, or microenvironmental factors.
Since the initial use of fat grafting by Neuber, graft resorption has remained a significant concern (13). Numerous volumetric analyses consistently reveal a relatively modest fat graft survival rate, ranging from 50 to 65% (14, 15). In clinical practice, Coleman applied his self-developed technique of structural fat grafting through facial infiltration in a cohort of 400 patients, reporting that grafts were effectively maintained over 6 years; however, graft preservation was assessed photographically (16).
In an experimental study, Schoeller et al. implemented 1 mL of preadipocytes into a fibrous capsule within the rectus muscles of rats and evaluated fat grafts at 1, 3, 6, and 12 months. He observed no fibrosis or abscess formation, and grafted fat volumes were sustained in all groups (17). Various theories, such as apoptosis due to insufficient vascularization of centrally located fat cells and trauma-induced damage to the grafts, have been proposed to explain graft resorption (18, 19). After adipose tissue transplantation, a hypoxic state persists until capillary circulation is established, with nutrition provided by diffusion from adjacent tissue during this period.
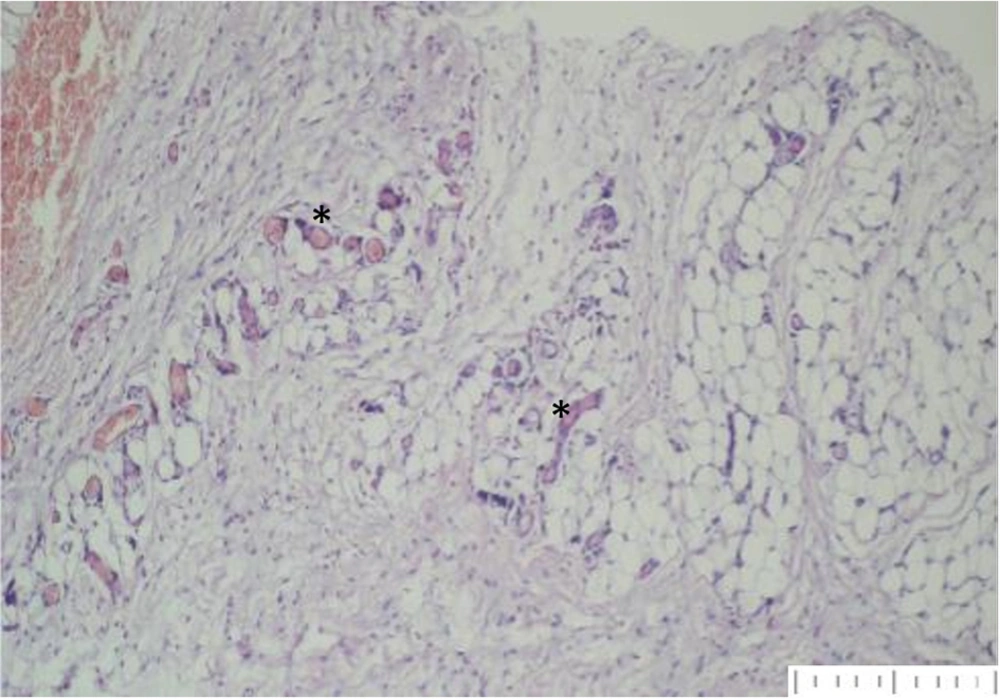
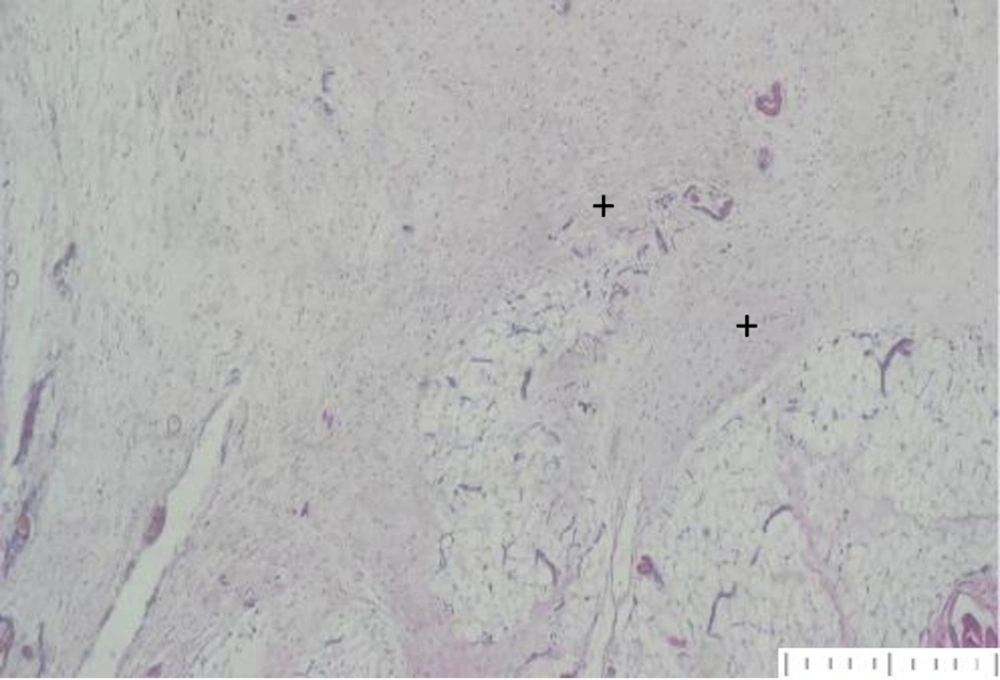
Yoshimura et al. found that while adipocytes are highly susceptible to hypoxia and die within 24 hours, adipose tissue-derived stem cells remain functional for up to 72 hours. Similarly, Zhu et al. determined that preadipocytes enhance graft retention and quality by promoting angiogenesis and preventing apoptosis through growth factor expression (20, 21). Our histologic evaluation confirmed the presence of well-organized vascularization around preadipocyte grafts (Figure 2) and fibrosis formation around adipocyte grafts (Figure 3).
5.1. Conclusions
Our study highlights the significant differences in the behavior of preadipocytes and adipocytes in grafting procedures, emphasizing the need for thorough assessment in cellular therapies. The findings reveal that grafted preadipocytes can sustain their volume over the duration of this experiment, whereas volumetric loss increased in adipocyte grafts. This suggests promising potential for autologous preadipocyte grafts in enhancing wound healing, addressing postoperative scars, and correcting congenital defects, including cleft palates, chest wall, and breast deformities (22, 23).
Given the challenges associated with fat grafts, particularly in pediatric patients with limited donor tissue, the use of adipose tissue-derived stem cells presents a transformative opportunity for soft tissue augmentation. Our results support further exploration into the application of preadipocytes in clinical practice, which may ultimately enhance efficacy and safety in regenerative medicine.