1. Background
Tissue expansion holds a significant place in the reconstructive surgeon's repertoire for addressing challenging and complex defects, particularly in aesthetically sensitive areas of the body. This versatile technique is used to manage defects arising from various etiologies, such as burn ulcers, contractures, traumatic chronic wounds, oncological excisions, congenital lesions like nevi and hemangiomas, meningomyelocele, and dermatological conditions like hidradenitis. Tissue expansion is particularly advantageous when distant or free tissue transfer is technically or logistically challenging.
Regionally, defects in cosmetically critical areas that cannot be closed primarily require reconstruction with tissues that closely match the surrounding area's color, texture, thickness, contour, and hair-bearing characteristics. Examples of such regions include the scalp, forehead, temporal region, malar areas, nose, neck, axilla, supraclavicular region, breast, and groin. These defects are often resurfaced using skin grafts or flaps from adjacent or distant parts of the body, which rarely achieve a satisfactory match in color and contour, making them cosmetically less acceptable.
The concept of tissue expansion has been widely adopted for surface defects due to its advantages, including simplicity, avoidance of donor site defects, and more aesthetically pleasing outcomes. However, the technique has its drawbacks, such as prolonged pain tolerance during serial expansions, the need for staged procedures, and the cost of the device. Additionally, several minor and major complications have been reported in the literature, including hematoma, seroma, remote valve exposure, inadequate expansion, cellulitis, infection, implant exposure, balloon deflation, and ischemic necrosis of the expanded skin (1-6).
The vascularity of expanded skin is crucial for its survival after advancement to cover the defect in the final stage of the procedure. During the serial inflation of expanders within the tissue pockets dissected in the initial stage, the overlying skin undergoes expansion while thinning in all its components. Therefore, it is essential to strike a balance between achieving adequate skin to cover the defect and limiting expansion to a safe extent.
There are various methods to assess the real-time vascularity of the skin, with Color Doppler Ultrasonography (a non-invasive technique) and CT angiography being among the more feasible options. Despite the availability of these techniques, no published reports have yet conclusively established the real-time increase in vascularity of expanded skin (7-11).
2. Objectives
Given the lack of evidence for real-time vascular assessment of expanded skin, this study aimed to evaluate changes in vascularity through radiological analysis and assess the safety and efficacy of tissue expansion in clinical applications. Correlating vascularity changes can help predict wound healing outcomes, minimize complications associated with wound healing, and determine the maximum safe limit for tissue expansion, enabling closures with reduced tension.
3. Methods
This study was an IEC-approved investigation (approval code: T/IM-F/21-22/09, granted on 08.04.2022), designed as a prospective, purposive, descriptive, non-randomized, comparative study and funded through an intramural research grant for a period of 12 months. The sample size was determined in accordance with the approved budget and conducted as a pilot study. A total of five remote-valve tissue expanders were procured for the study, deemed appropriate for three patients who were selected based on specific inclusion criteria. The inclusion criteria included patients with defects arising from the excision of post-burn ulcers, scars, or contractures located in major functional or cosmetic areas such as the breasts, scalp, and neck. Patients aged between 12 and 60 years were included, provided they did not have significant comorbid conditions, including uncontrolled diabetes, hypertension, cardiac failure, collagen vascular diseases, chronic dermatological illnesses, psychiatric disorders, or seropositive status. The study endpoint was defined as the completion of the second stage, during which the expander was removed, the flap advanced to reconstruct the post-excision defect, and the wound subsequently healed. The objectives of the study were to evaluate changes in the vascularity of the expanded skin using radiological methods and to determine a safe extent of tissue expansion that minimizes the risk of flap necrosis or failure.
After a session of counseling, informed consent was obtained from every patient included in the study. Depending on the site, size, nature, and extent of the defect, planning was carried out. On admission, Colour Doppler study and CT-angiography of the adjacent local tissues were performed to document the vascularity of the region. The area of skin planned for expansion was marked, and its vascularity was assessed in terms of intravascular flow velocity, waveform, lumen diameter, vessel density, and feeders with the help of an 11 MHz Colour Doppler with Power Doppler mode and a CT-angiography simultaneously, using the dye Iohexol (256 slices with 1 mm cuts). As the blood flow is reflected in the Colour Doppler with high frequency (> 7 MHz probes), it distinguishes venous and arterial blood flow movements, while the Power mode indicates the volume of flow simultaneously, which in turn provides a real-time status of the cutaneous vascularity, comparing pre- and post-expansion changes in the same region. Also, CT-angiography provides an accurate estimate of the lumen diameter of the major feeding vessels and their density in the pre- and post-expansion settings. Hence, it helps in assessing the vessel density and the dimensions with better accuracy (7-11).
These two modalities of radiological investigations were incorporated in this study simultaneously for both subjective as well as objective evaluation of increased vascularity (neovascularization) in the expanded tissue. Increased vascularity was assessed in terms of the number of blood vessels in a square centimeter area of skin subjected to expansion. These parameters were recorded and saved for comparison with those observed after the completion of expansion. After tabulating the data in Microsoft Excel (2013), it was analyzed with Stata 12 software. The comparison between different parameters was tested by the Mann-Whitney U test, considering a P-value < 0.05 to be statistically significant (Table 1).
| Radiological Parameters with Units and Case no. with Anatomical Details of Vessels Evaluated Radiologically | Pre-expansion | Post- expansion | P-Value b |
|---|---|---|---|
| 1. Vessel velocity (cm/sec) as per Colour Doppler | 0.461 | ||
| Case no. 1 | |||
| Rt. IMA | 15 | 11 | |
| Lt. IMA | 9 | 12 | |
| Case no. 2 Lt. STA | 7.7 | 21 | |
| Case no. 3 Rt. ECA | 11 | 14 | |
| 2. Lumen diameter (in mm) as per Colour Doppler | 0.500 | ||
| Case no. 1 | |||
| Rt. IMA | 2.5 | 2.3 | |
| Lt. IMA | 2.4 | 2.3 | |
| Case no. 2 Lt. STA | 0.6 | 0.8 | |
| Case no. 3 | |||
| Rt. ECA | 1.7 | 1.9 | |
| Rt. IMA | 1.2 | 1.5 | |
| 3. Lumen diameter (in mm) as per CT-angiography | 0.017 | ||
| Case no. 1 | |||
| Rt. IMA | 1.8 | 2 | |
| Lt. IMA | 1.9 | 2.2 | |
| Case no. 2 | |||
| Lt. STA (Frontal) | 1.2 | 1.3 | |
| Lt. STA (Parietal) | 1 | 1.4 | |
| Lt. STA (Occipital) | 1.5 | 1.7 | |
| Case no. 3 | |||
| Rt. ECA | 1.8 | 2 | |
| Rt. IMA | 1.5 | 1.8 | |
| 4. Density of vessels (per square cm) as per Colour Doppler | 0.065 | ||
| Case no. 1 | |||
| Rt. IMA | 0.2 | 0.4 | |
| Lt. IMA | 0.2 | 0.4 | |
| Case no. 2 Lt. STA | 1.5 | 3 | |
| Case no. 3 Rt. ECA | 0.2 | 0.3 | |
| 5. Major feeder vessels (anatomical) as per CT-angiography | Qualitative variable | ||
| Case no. 1 | Bilateral Internal Mammary Artery (IMA) | Bilateral IMA | |
| Case no. 2 | Lt. Superficial Temporal Artery (STA)- Frontal, Parietal, Occipital branches. | Lt. STA and its branches | |
| Case no. 3 | Branches of Rt. External carotid artery (ECA) and Rt. IMA | Branches of Rt. ECA and IMA | |
| 6. Waveform as per Colour Doppler | Qualitative variable | ||
| Case no. 1 | Monophasic | Biphasic | |
| Case no. 2 | Monophasic | Biphasic | |
| Case no. 3 | Monophasic | Biphasic |
a After tabulating the data in Microsoft Excel (2013), it was analysed with Stata 12. The comparison between different parameters was tested by Mann- Whitney U test, considering P-value < 0.05 to be statistically significant.
b Statistical analysis (using Mann-Whitney U test for paired non-parametric continuous data using Stata 12 software).
3.1. Surgical Technique
The area of adjacent skin designated for expansion was initially marked. This area was infiltrated with an adrenaline/normal saline solution (1:100,000 dilution) in a volume appropriate for the patient’s body weight. The incision was planned carefully, considering the direction of the advancement of the expanded flap and ensuring the safe dissection of the pocket for the expander balloon. The incision was either placed adjacent to the lesion, where the leading edge of the advancement flap would lie, or within the lesion, ensuring a secure wound closure.
The plane of dissection was chosen to be a relatively avascular layer over the epimysium of the muscle for most areas of the body, and sub-galeal in the scalp and forehead. Meticulous haemostasis was achieved before introducing the expander into the dissected tissue pocket. The expander's remote port was placed at a distant site. The pocket was irrigated with povidone-iodine or an antibiotic solution, and the expander was positioned comfortably without tension.
Once placed, the deflated expander was inflated with a test dose of normal saline injected through the distant port to confirm the absence of leaks and the integrity of the implant. The saline was then withdrawn, deflating the expander, and the surgical site was closed over suction drains in layers. After closure, the expander was re-inflated with normal saline to obliterate any dead space within the pocket, maintain the pocket size, and smooth out wrinkles or folds in the expander envelope (1).
Postoperatively, the patient was closely monitored, and the wound was inspected regularly during the initial days until discharge. Broad-spectrum antibiotics were administered during this period. Drains were removed once the output decreased to an insignificant amount. The wound was allowed to heal over 2 - 3 weeks before beginning the expansion process, which was conducted on an outpatient basis. The limit of each expansion session was determined by the patient’s tolerance to pain or discomfort resulting from acute expansion, with sessions conducted at weekly intervals. Sutures were removed approximately three weeks after surgery.
Upon completion of the expansion process, patients were re-admitted for excision of scars, removal of the implants, and advancement of the expanded skin flap to resurface the defects.
A repeat Color Doppler and CT-angiography were conducted, as previously described, before the removal of the expander implant, and the parameters were compared with those recorded prior to expansion.
The expander was removed through the original incision, and the edges of the expanded flap were freshened. Scars were excised, and the expanded skin flaps were advanced to reconstruct the scarred region. Closure was performed in layers over a drain. Postoperatively, the patient was monitored closely for several days under antibiotic coverage and followed up at weekly intervals for one month after discharge.
Complications arising during surgery, the expansion process, or following the advancement of the expanded flap—such as surgical site infections, cellulitis, disproportionate pain, expander rupture, or extrusion—were promptly addressed and documented.
The results of this study were qualitatively interpreted based on observed changes in vascularity and the clinical outcomes. Clinical outcomes were evaluated using parameters such as wound healing, defect coverage, aesthetic results, and complications.
4. Results
A total of 5 expanders were used in 3 patients over a one-year period (December 2022 to December 2023). Of these, 1 expander, placed in a patient with bilateral supraclavicular region expansion, failed due to infection and extrusion of the implant. The remaining 4 expanders successfully achieved the surgical goals of resurfacing and were included in the study, yielding a success rate of 80% for the tissue expanders. Comparative analysis of radiological evaluations (post-expansion versus pre-expansion) revealed an increase in all parameters assessing the vascularity of the skin flaps. There was a marked increase in neovascularization, with a higher number of feeder vessels, increased vessel density, enhanced flow velocity, and improved flow reaching the advancing edges of the flaps (Table 1).
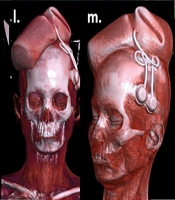
The first patient (Case 1) presented with post-burn symmastia and underwent tissue expansion of the suprasternal area using one croissant-shaped tissue expander. Six sessions of expansion were performed to successfully resurface the breast cleavage area, with definitive advancement of the expanded skin between the breasts (Figure 1).
a, planning for tissue expansion of a 24-year-old female with post-burn scar on breasts & symmastia; b, pre-expansion colour doppler; c & d, pre-expansion CT angiography; e, pre-operative planning & rationale of 1 croissant shaped expander in pre-sternal area estimating area of advancement in terms of volume and dimensions of the expander (croissant 250 cc, 14.5 × 7.7 × 4.5 cm); f, expander inserted after creating the pocket; g, early post-operative image after 3 days; h, after 6 sessions of expansion (240 cc) over 2 months; i, post-expansion colour doppler; j & k, CT- angiography with 3-dimensional reconstruction post-expansion; l, removal of expander through excision of scar in the pre-sternal and inter-mammary area; m, excised scar; n, expander removed with remote port; o, expanded skin advanced inferiorly and wound closed creating adequate inter-mammary area; p, early post-operative image after 7 days.
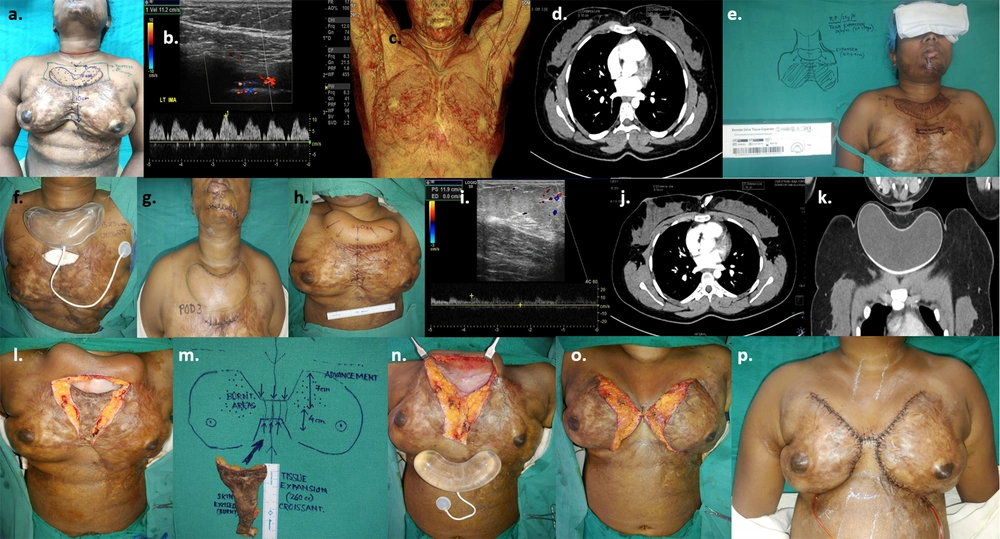
The second patient (Case 2) had a post-burn scar with alopecia on the left fronto-temporal area of the scalp. Two expanders, one cylindrical and the other rectangular, were introduced. After seven sessions of expansion, the expanders were removed, the scar was excised, and the defect was successfully resurfaced with the expanded hairy skin flap (Figure 2).
a, 19-year-old girl with post-burn scar & alopecia scalp; b, pre-expansion colour doppler; c, pre-expansion CT angiography; d, pre-operative planning of 2 expanders estimating area of advancement in terms of volume and dimensions of the expanders (cylindrical on rt. side of scalp- 250 cc, 10.2 × 5.4 × 7.2 cm and rectangular on lt. side of scalp- 300 cc, 10 × 6 × 6.3 cm); e & f, expanders inserted after creating sub-galeal pockets; g, closure of wound; h & i, early post-operative image after 1 week; j & k, After completion of expansion (240 cc on right side & 280 cc on left side) over 4 months; l & m, 3-dimensional reconstruction of CT images post-expansion; n, colour doppler post-expansion; o, to q, expander removal and definitive wound closure after excision of alopecic scar 18 × 8 cm (Figure 2. r. inset); s & t, late post-operative results after 3 months.
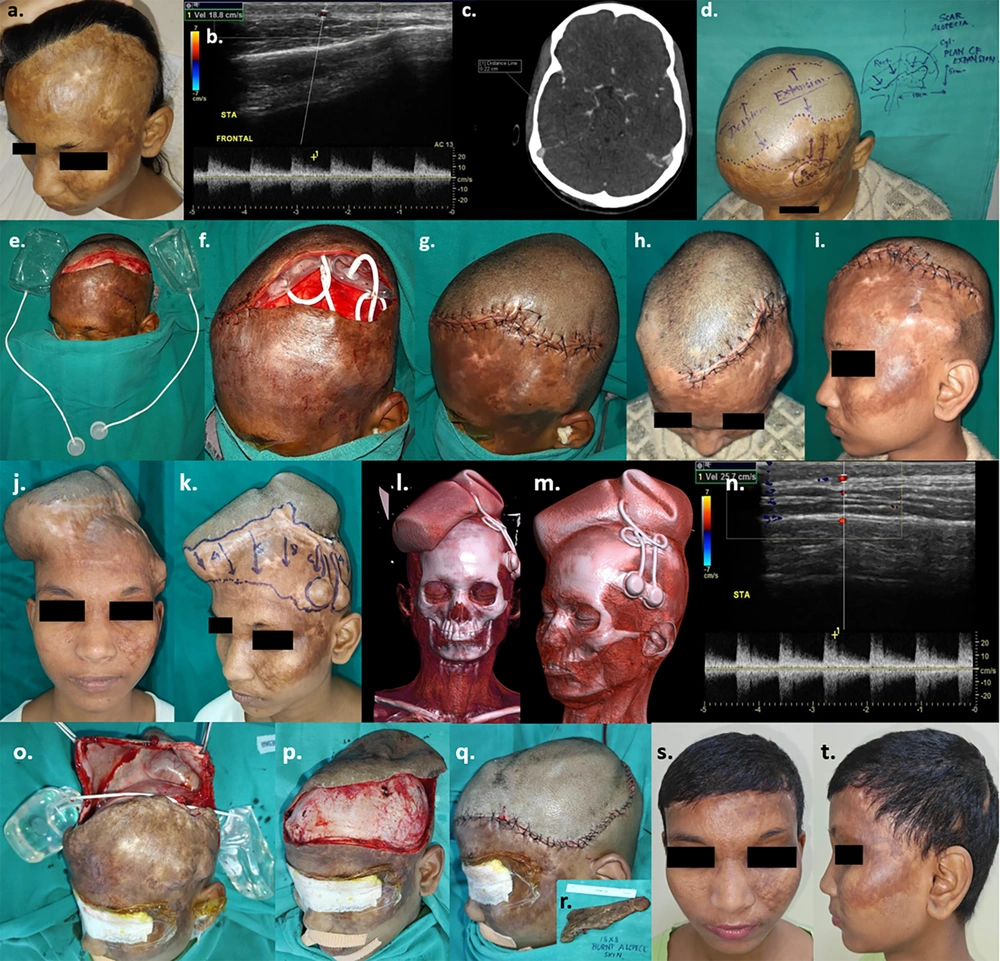
The third patient (Case 3) presented with a post-burn contracture involving the neck and upper chest. Two expanders, one cylindrical and one rectangular, were introduced into the bilateral supraclavicular areas. However, one expander failed due to infection and was extruded. The extruded expander in the left side of the neck was surgically removed. Resurfacing of half the defect was achieved with the expanded flap, while the remaining area was skin grafted (Figure 3).
a to c, Planning for tissue expansion of a 23-year-old female with post-burn neck contracture (Grade III) and upper chest wall hypertrophic hypopigmented scar; d, pre-expansion colour doppler; e & f, pre-expansion CT angiography; g & h, insertion of 2 expanders estimating area of advancement in terms of volume and dimensions of the expanders (cylindrical on rt. supraclavicular region- 250 cc, 10.2 × 5.4 × 7.2 cm and rectangular on lt. supraclavicular region- 300 cc, 10 × 6 × 6.3 cm); i, early post-operative images after 2 weeks; j & k, left sided implant extrusion due to infection & suture dehiscence after 4 weeks following which left sided expander removed; l to n, after 7 sessions of expansion (280 cc) on the right sided expander over 4 months; o, post-expansion colour doppler; p, post-expansion CT-angiography with 3-dimensional reconstruction; q, Excision of post-burn scar and removal of expander; r to t, defect on neck & upper chest after excisional release and advancement of right sided supraclavicular expanded skin; u, defect resurfaced with intermediate thickness split skin graft; v, early post-operative image - 5th post-operative day; w, 4 weeks post-op ; x to z, 3 months post – op with adequate neck extension & cervico-mental angle.
Statistical analysis of the results showed that, among all the parameters, the lumen diameter (measured in mm) assessed via CT-angiography before and after expansion was found to be statistically significant (Table 1).
5. Discussion
Human skin, owing to its viscoelastic properties, has the ability to gradually expand and stretch over time under both physiological and pathological conditions. Codvilla (1, 2) first reported femoral elongation using bony traction in 1905. Neumann in 1957 reconstructed part of the external ear using a latex balloon, a technique further developed by Radovan in 1976 for addressing arm defects (1-6). This unique property of the skin can be utilized to cover and resurface defects caused by various etiopathologies (where primary closure is not possible) by subjecting adjacent healthy skin to mechanical stress through gradual expansion (1, 2).
Mechanical stress applied to living skin and subcutaneous tissues activates several integrated signaling pathways involving the cytoskeletal system, extracellular matrix, enzyme activation, secondary messengers, and ion channels. This process triggers the release of cytokines, chemokines, growth factor expression, matrix metalloproteinase expression, and both anti-inflammatory cytokines (IL-10) and pro-angiogenic growth factors (VEGF, FGF, PDGF), as demonstrated in animal models (2). These biochemical changes promote neovascularization, leading to increased vascularity in the expanded tissue. Enhanced vascularity improves the survival of the expanded skin when it is advanced a significant distance as a pedicled flap for reconstructive purposes.
Following skin expansion, notable changes have been observed, including an increase in epidermal thickness, heightened melanocytic activity, distortion of hair follicles, and a decrease in dermal thickness. A dense, fibrous capsule forms around the expander, with collagen deposition and the development of an extensive vascular plexus within the capsule. In cases where the expander is placed in a submuscular plane, underlying muscles exhibit atrophy, muscle fiber degeneration, glycogen deposition, and interstitial fibrosis. Additionally, there is observable expansion of the underlying bones, particularly cranial bones, accompanied by a reduction in their thickness and volume (1, 2, 12).
There is an increase in the vascularity of expanded tissue, which is believed to be the basis for the survival of the distal flap margin after it is advanced. However, this increase in vascularity has not been extensively studied in real-time settings. Our study aimed to establish this fact and successfully provided evidence through Color Doppler imaging and CT angiography. Color Doppler enhances the specificity of sonographic imaging, enabling real-time evaluation of vascularity (7). Several studies have emphasized the diagnostic utility of Color Doppler imaging in evaluating vascularity in dermatological lesions (8-10). Additionally, a cadaveric study has investigated subdermal and cutaneous vascular perfusion, including the predominant direction of circulation, anastomosis, and perfusion volume, using CT angiography (11).
Following expansion, a significant number of new blood vessels form adjacent to the capsule. Initially, the collagen fiber content in existing vessels decreases, while elastic fibers increase, alongside a rise in vascular endothelial growth factor (VEGF) levels, which promotes increased supra-fascial vascularity (1, 2). Mechanical stress induces angiogenesis, leading to enhanced vascularity in the distal peripheral areas of the flap, thereby offering functional benefits.
We preferred conventional tissue expanders with a remote injection port due to their better safety profile and cost-effectiveness. Expanders with integrated ports, where the injection port is incorporated directly into the prosthesis, carry a risk of inadvertent perforation while localizing the port during saline injection. Additionally, repeated injections near the implant increase the risk of infection. Remote-port expanders mitigate these risks. Recently, self-inflating expanders containing osmotic hydrogel have been developed. These devices facilitate the migration of extracellular water through the silicone membrane, enabling progressive enlargement and tissue expansion without requiring repeated injections. This reduces patient discomfort and minimizes infection risks (2). However, these expanders are expensive and not widely available.
Meticulous planning is crucial before the surgical introduction of implants (1-4). Incisions should be designed to align with the future margins of the flap, whether for advancement, rotation, or transposition. Aesthetic units should be respected, with scars placed in minimally conspicuous locations, and the suture line should be free from undue tension. Achieving meticulous hemostasis during and after tissue pocket dissection is essential to prevent hematoma. The dissected pocket must be sufficiently sized to comfortably house the expander and should be located in a relatively virgin, well-vascularized area. Initial inflation tension is generally greater when incisions are parallel to the direction of expansion compared to perpendicular incisions. The injection port should be placed as remotely as possible from the expander.
Regarding implant size, selecting an implant equal to or slightly smaller than the donor area is recommended. In some cases, multiple expanders may be used (1, 2). In one of our cases, complications arose in the form of infection and implant exposure. The patient had two expanders inserted in the neck to resurface a post-burn scar. Unfortunately, one of the implants became infected, necessitating its removal—an outcome not uncommon in such cases.
Tissue expanders have seen a broad range of applications in recent years, revolutionizing the reconstruction and resurfacing of defects, particularly in the face, scalp, and neck, where it is prudent to reconstruct “like with like”. However, expansion carries a higher rate of complications in children and must be used cautiously (2, 3). In planned reconstructions with myocutaneous, fasciocutaneous, and free flaps, the use of expanders significantly increases the flap territory. Even for full-thickness skin grafts, pre-harvest expansion can increase the size of the graft.
In the head and neck regions, tissue expansion is particularly valuable for specialized areas such as the hair- and non-hair-bearing regions of the scalp, forehead, temple, nose, malar, and periorbital areas. Tissue expanders also play a pivotal role in reconstructing post-mastectomy defects where large implants cannot initially be accommodated. They are frequently used in delayed breast reconstruction or cases of hypomastia, where the skin envelope is insufficient to accommodate an implant. In cases of irradiated chest walls, where vascularized tissue is scarce, tissue expansion proves beneficial.
Tissue expanders are also effective for addressing marked chest wall deformities in Poland syndrome and for reconstructing areas affected by giant hairy nevi, particularly on the trunk. Post-traumatic and post-burn defects of the extremities can also benefit from tissue expansion, demonstrating its versatility in various reconstructive scenarios (1, 2).
Our study was a pilot project, limited by its very small sample size. However, it aims to pave the way for future research to strengthen the evidence by incorporating larger sample sizes. The radiological parameters assessed in this study were both quantitative and qualitative in nature. Future studies could focus on integrating more extensive quantitative data to derive statistical significance, thereby better establishing the findings.
In summary, tissue expansion leads to increased vascularity, which forms the foundation for the survival of advanced expanded skin flaps. This technique can be considered safe and effective for reconstructing cosmetically significant areas of the body, particularly for defects resulting from the excision of benign or congenital lesions. Changes in the vascularity of the skin can be reliably assessed using imaging modalities such as color Doppler and CT angiography with specific radiological parameters. These tools enhance our ability to evaluate the safety and efficacy of tissue expansion for reconstructive procedures.