1. Introduction
The most common cause of diffuse, non-scarring hair shedding is Telogen Effluvium (TE). It does not appear to have any racial or ethnic preference but is more commonly observed in females (1). Telogen effluvium may present as acute or chronic hair loss, sometimes accompanied by symptoms such as trichodynia (2). Acute TE is more frequent than its chronic variant, which is typically diagnosed in women aged 30 to 60 years (3, 4). Chronic TE is characterized by an insidious onset of diffuse hair loss that persists for more than six months.
Telogen effluvium is usually diagnosed based on patient history and trichoscopic examination, while laboratory analysis can help identify unclear triggers. In the absence of concurrent hair or scalp disorders, the scalp and hair shafts often appear normal, with minimal variations in hair shaft diameter, and symptoms may be mild or absent. Effective management of TE requires identifying and addressing the underlying cause. Although some clinicians prescribe topical minoxidil for treating Chronic TE, evidence for its efficacy remains limited. Minoxidil is generally used to stimulate hair regrowth and maintain hair density; however, few effective treatment options exist for this condition (2, 5).
In recent years, microneedling has gained attention as a potential treatment option. This technique creates tiny transdermal channels through the stratum corneum—the skin's outer barrier layer—enhancing the absorption of small-molecule medicines, proteins, and even vaccines. Microneedling stimulates the production of key structural components of the skin, such as collagen, elastin, and proteoglycans, making it effective for treating various skin conditions, including photoaging, wrinkles, scars, pigmentation changes, alopecia, and vitiligo. Its high efficacy, minimal side effects, and short recovery time have made microneedling a popular choice in both cosmetic and medical treatments (6). Additionally, microneedling offers more reliable drug delivery than oral administration by bypassing digestive breakdown and liver metabolism.
A variant of microneedling, known as Microinfusion of drugs into the skin (MMP®), was introduced by Arbache and Godoy. This technique uses a tattoo machine to deliver medications into the skin, potentially enhancing the therapeutic effects of treatments like those for hair loss. While some case reports show promising results, conclusive data supporting MMP®'s effectiveness in treating TE and other hair disorders are still lacking (7).
2. Case Presentation
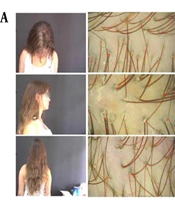
In our study on MMP® with minoxidil for female pattern alopecia, a 28-year-old patient with a three-year history of hair loss and reduced hair volume participated. She had a history of polycystic ovary syndrome, was using oral contraceptives (chlormadinone + ethinylestradiol), and had a family history of female pattern alopecia (mother and sister). Trichoscopy, conducted with the assistance of TrichoLAB® software, confirmed the diagnosis of chronic telogen effluvium (CTE) (Figure 1A).
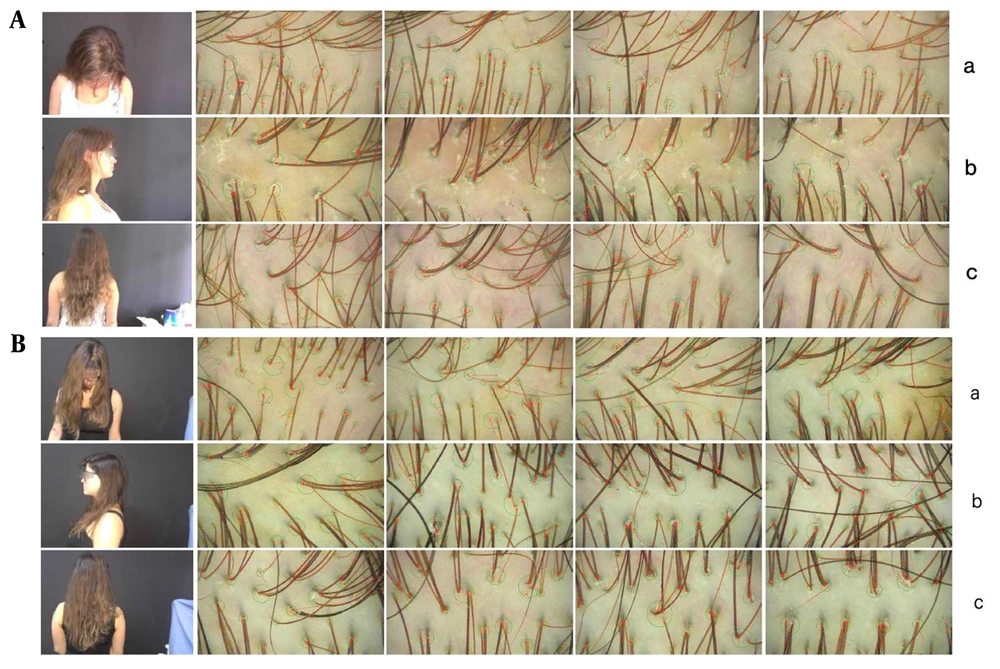
A, clinical and trichoscopic image of the patient before treatment with MMP® with 0.5% minoxidil (Health Tech, Anvisa 0094729); (a) frontal region; (b) parietal-vertex region; (c) occipital region (images obtained by TrichoLAB®). B, clinical and trichoscopic image of the patient after 3 monthly sessions of MMP® with 0.5% minoxidil (Health Tech, Anvisa 0094729); (a) frontal region; (b) parietal-vertex region; (c) occipital region (images obtained by TrichoLAB®).
Although minoxidil use for TE is not fully supported by direct scientific evidence in this context, the patient provided informed consent, which was approved by the regional ethics committee. We conducted extensive evaluations to exclude systemic diseases, nutritional deficiencies, infections, and any potential drug or endocrinological causes for her hair loss.
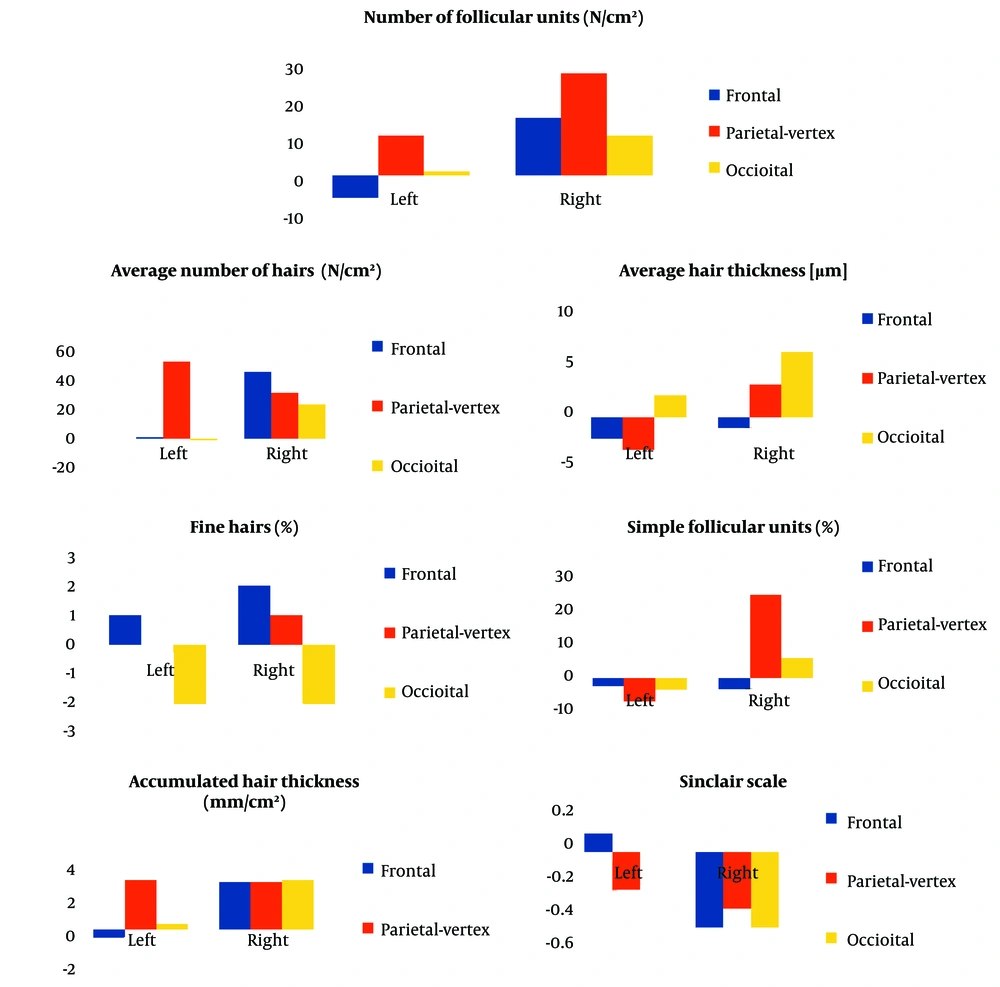
The treatment protocol involved three monthly sessions of MMP® using 0.5% minoxidil on the right side of the scalp (frontal-parietal-vertex regions). After each session, the scalp was occluded with plastic film for 12 hours. Additionally, the patient was prescribed a 5% minoxidil solution for daily home application on both sides of the scalp, starting 72 hours post-intervention to allow for closure of the microneedling microchannels. Six weeks after the final session, a trichoscopic reassessment of the scalp was performed (Figure 1B), and the patient completed a Self-Assessment Questionnaire. Pre-treatment and post-treatment TrichoLAB® statistics revealed the following results which has been shown in Figure 2.
2.1. Frontal Region
- Average Hair Density: Hair density increased slightly in the treated region, from 206 to 207 hairs, while it remained stable on the untreated side.
- Hair Shaft Thickness: A decrease in hair shaft thickness was observed in both regions, with the treated area decreasing by 2 µm (from 61 µm to 59 µm) and the untreated side showing a similar reduction of 2 µm.
- Follicular Units: The treated side showed a decrease of 2 follicular units containing single hairs, while the untreated side remained unchanged. Additionally, the fine hair count on the treated side dropped by 1 hair, with no change observed on the untreated side.
2.2. Parieto-Vertex Region
- Average Hair Density: The treated side showed a significant increase in hair density, rising from 223 to 270 hairs, a gain of 47 hairs. The untreated side also experienced an increase of 28 hairs.
- Hair Shaft Thickness: Hair shaft thickness in the treated area decreased by 3 µm (from 67 µm to 64 µm), while the untreated area saw an increase of 3 µm (from 65 µm to 68 µm).
- Follicular Units: The treated side showed an increase in follicular units containing single hairs, while the untreated side showed a decrease. Additionally, the treated side exhibited an increase of 12 follicular units containing three or more hairs, compared to a slight reduction on the untreated side.
2.3. Occipital Region
- Average Hair Density: Hair density decreased slightly in the treated area by 1 hair (from 211 to 210), while the untreated side experienced an increase of 21 hairs (from 199 to 220).
- Hair Shaft Thickness: Hair shaft thickness in the treated area increased by 2 µm (from 58 µm to 60 µm), while the untreated area showed a greater increase of 6 µm (from 54 µm to 60 µm).
- Follicular Units: The treated region saw a decrease of 3 follicular units containing single hairs, while the untreated side showed an increase in units containing more than two hairs.
3. Discussion
The study employed a split-scalp design with a 16-week protocol to differentiate initial responses from the chronic effects of topical minoxidil, which typically requires at least four months to produce noticeable results (8). Minoxidil is thought to promote hair growth by extending the anagen phase and reducing the telogen and kenogen phases, thereby increasing the thickness of miniaturized follicles in Female Pattern Alopecia (9). However, its specific efficacy for TE has not been thoroughly evaluated in clinical studies.
Experts frequently prescribe minoxidil for CTE to preserve hair density and stimulate growth, though continuous use may be required to maintain benefits (2, 5, 9). According to the patient’s self-assessment, she observed a slight increase in hair volume, although hair shedding remained unchanged. Pain was manageable (rated 5 on a Pain Scale), and no hair shaft breakage was observed—a common issue with other microneedling devices (10).
MMP® offers multiple potential benefits, including the release of growth factors and the activation of stem cells in the hair bulb, both of which are essential for hair growth induction. These effects are similar to those observed with platelet-rich plasma treatments (11). Future studies, including prospective double-blind, half-head trials, are needed to confirm these preliminary findings and further explore the efficacy of MMP® combined with minoxidil in managing CTE, a condition that significantly affects patients' quality of life (2).
Our study observed key trends in hair density and thickness, essential for assessing the efficacy of MMP® combined with minoxidil in treating CTE. For instance, there was a noticeable increase in hair density on the treated side (from 206 to 207 in the frontal region and from 223 to 270 in the parieto-vertex region), suggesting that MMP® may improve minoxidil absorption and effectiveness.
The split-scalp design allows for a direct comparison between treated and untreated areas, providing a robust method to demonstrate the localized effects of the treatment. The observed increases in hair thickness and changes in hair counts across different hair types (fine, medium, and thick) further support our hypothesis that MMP® enhances drug delivery and may stimulate hair follicle activity.
The potential mechanisms by which MMP® amplifies minoxidil’s effectiveness are rooted in molecular pathways influenced by both treatments. MMP® is known to trigger natural healing processes, including the activation of various growth factors. Specifically, the Wnt signaling pathway plays a critical role in hair follicle development and cycling, with the Wnt3a and Wnt10b genes promoting hair follicle neogenesis and transitioning follicles into the anagen (growth) phase (12). Additionally, minoxidil’s ability to extend the anagen phase is likely enhanced by MMP®, which increases scalp permeability and drug delivery. Enhanced expression of vascular endothelial growth factor (VEGF) following MMP® treatment also promotes increased follicular blood flow, supporting hair growth and follicle health (13).
Thus, the combination of MMP® and minoxidil not only promotes hair growth through traditional pathways enhanced by minoxidil but also utilizes microneedling-induced upregulation of key signaling molecules critical for effective hair regeneration.