1. Context
Skin problems, though not life-threatening, can significantly impact healthcare systems and quality of life. Genetics and socioeconomic factors influence their prevalence and severity. Challenges include high treatment failure rates and the contagious nature of certain conditions. Common skin issues include acne, wrinkles, skin cancer, heat rashes, and hypopigmentation (1). This review elaborates on various dermatological disorders and their respective remedies.
2. Evidence Acquisition
Acne, a common skin disorder, affects 85% of adults, particularly adolescents, and is influenced by factors such as genetics, stress, androgens, and excessive sweating. Increased sebum production due to androgenic stimulation during adrenarche leads to sebaceous gland enlargement, contributing to acne severity (2, 3). Cutibacterium acnes (formerly P. acnes) colonizes sebaceous follicles, breaking down sebum and triggering inflammation. This process promotes the formation of comedones and skin irritation. Inflammatory mediators, such as cytokines regulated by NF-κβ, and lipid mediators like COX-2, exacerbate acne by stimulating keratinocyte proliferation and attracting inflammatory cells to the skin (4, 5).
2.1. Clinical Classification of Acne
Grade I acne includes whiteheads, blackheads, and a few papules and pustules, while grade II features numerous papules and pustules. Grade III, or nodulocystic acne, involves inflamed nodules, and grade IV is characterized by painful, inflamed pustules.
Different lines of treatment for acne are depicted in Table 1.
| Types of Acne | Primary Therapy | Secondary Therapy | Tertiary Therapy | Hormonal Based Solution for Women |
|---|---|---|---|---|
| Comedonal acne | Topical retinoids, adapalene, isotretinoin | Azelaic acid | - | - |
| Mild to moderate papulo pustular acne | Benzoyl peroxide + adapalene | Azelaic acid + adapalene | Tretinoin + topical erythromycin + azelaic acid + benzoyl peroxide + systemic antibiotics | - |
| Severe papulo pustular acne, mild nodular acne | Systemic isotretinoin | Systemic antibiotics + benzoyl peroxide + adapalene + azelaic acid | - | Hormonal anti androgens + topical treatment |
| Severe nodular, conglobate acne | Systemic isotretinoin | Systemic antibiotics + azelaic acid | Systemic antibiotics + benzoyl peroxide | Hormonal antiandrogens + systemic antibiotics |
2.2. Pigmentation
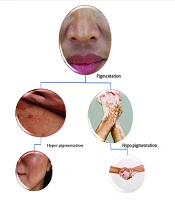
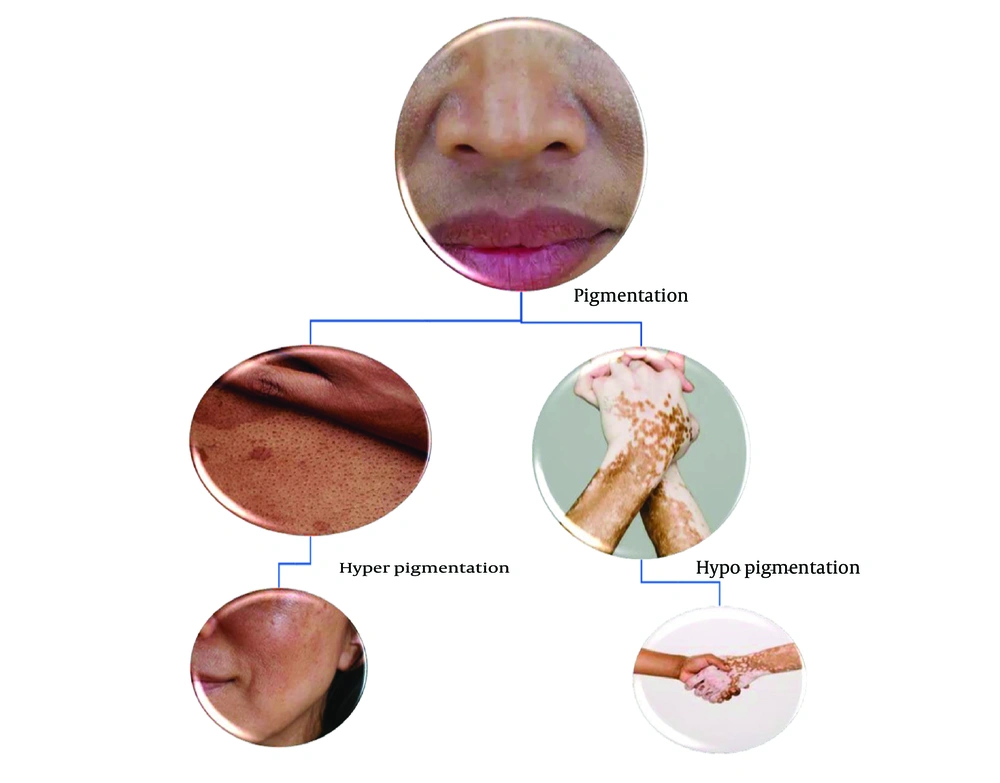
Pigmentation is regulated by melanin produced by melanocytes, with tyrosinase catalyzing its formation and influencing skin color through eumelanin or pheomelanin. Genes such as MC1R regulate the amount and type of melanin produced, thereby affecting skin color and pigmentation (6, 7). Hyperpigmentation results from excess melanin, while depigmentation is caused by melanin loss, often triggered by UV radiation (8). Pigmentation disorders include depigmentation (e.g., vitiligo), hypopigmentation, and hyperpigmentation (9), as illustrated in Figure 1.
Vitiligo has two main forms: Non-segmental, which affects both sides of the body, and segmental, which affects one side and stabilizes over time. Treatment options for vitiligo include phototherapy and topical creams designed to stimulate pigment production (10-13).
Topical treatments for pigmentation include:
- Topical steroids: Etnovate, Diprolene-cream, lotion.
- Topical anti-infectives: Silvasorb, Solox-cream, gel.
- Topical steroids with anti-infectives: Yaliika Pak, Triheal-80 -cream.
- Topical depigmenting agents: Tri Derma, Tri Luma-cream (14).
Herbal treatments for pigmentation focus on inhibiting tyrosinase, the enzyme responsible for melanin production (15). Key herbs such as turmeric, liquorice, Aloe vera, and mulberry extract reduce melanin synthesis and lighten dark spots, while vitamin C from Amla protects against UV-induced pigmentation. These herbs provide anti-inflammatory and antioxidant benefits in addition to their skin-lightening effects (16-19).
2.3. Wrinkles
Wrinkles are categorized into fine and coarse types. These are primarily caused by aging, reduced skin elasticity, and UV damage, which breaks down collagen and elastin. Repeated facial expressions and genetics also contribute to their development (20, 21). Wrinkles appear as horizontal lines, crow's feet, and laugh lines, with dynamic wrinkles forming due to facial movements and static wrinkles resulting from a loss of elasticity. Treatment options for wrinkle reduction and skin rejuvenation include retinoid creams, microdermabrasion, chemical peels, Botox® injections, hyaluronic acid fillers, and facelift surgery (22).
Herbal treatments for wrinkles focus on boosting collagen production and skin elasticity while providing antioxidant protection. Aloe vera enhances hydration and collagen synthesis, while green tea protects against oxidative damage and supports collagen formation (23, 24). Rosehip oil regenerates collagen, ginseng improves skin elasticity, and turmeric prevents collagen breakdown through its anti-inflammatory effects. Together, these herbs reduce fine lines and improve skin texture (25-27).
2.4. Prickly Heat
Prickly heat (miliaria) is primarily caused by sweating, exacerbated by heat, humidity, physical activity, and certain medications such as neostigmine and bethanechol, as well as conditions like Morvan syndrome and pseudohypoaldosteronism (28-32). Symptoms include small bumps, itching, prickling sensations, and discomfort, often occurring in skin folds or areas where the skin rubs against clothing. It is intensified by elevated body temperatures and environmental factors (33) (Table 2).
| Types | Size | Causes |
|---|---|---|
| Miliaria crystalline | Appears as small bumps. Bumps with size 1 - 2 mm | No itching and pain, seen only in babies and adults |
| Miliaria profunda | Occurs in deeper layers of skin | Causes relatively large, tough, flesh-coloured bumps |
| Miliaria rubra | Occurs in deeper layers of skin and more uneasy | Causes larger bumps, inflammation, and a lack of sweat in the affected area |
Prickly heat can be prevented by limiting heat exposure, regular gentle exfoliation, and taking cool showers to avoid sweat gland blockages. Patting the skin dry gently helps reduce irritation and the risk of rashes (34).
Herbal treatments for prickly heat focus on soothing inflammation, reducing sweating, and providing antimicrobial benefits. Aloe vera offers cooling and anti-inflammatory effects, while neem helps prevent infection with its antibacterial properties (35, 36). Chamomile reduces irritation and calms the skin, and Tulsi (Holy Basil) cools and heals the skin with its antioxidants. Together, these herbs help alleviate symptoms, prevent infection, and promote skin healing. These natural remedies are effective for managing prickly heat (37, 38).
2.5. Skin Cancer
Skin cancer is one of the most common and rapidly increasing cancers, often occurring on sun-exposed areas, leading to significant health and healthcare costs. Treatment options include surgery, cryotherapy, chemotherapy, immunotherapy, and radiation, with prevention focusing on sun safety measures such as the use of sunscreen (39).
2.5.1. Etiology
The risk of developing basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) is strongly linked to cumulative UV exposure over a lifetime. Melanoma, the most lethal form of skin cancer, originates from mutated melanocytes. Unlike BCC and SCC, the risk of melanoma is more closely associated with sun exposure during adolescence. Risk factors for skin cancer also include a family history of the disease, chemical exposure, tanning bed usage, HPV infection, Fitzpatrick skin type, the presence of melanocytic nevi, and immunosuppression.
2.5.2. Types of Skin Cancer
2.5.2.1. Basal Cell Carcinoma
It originates in the upper layer and basal cells of the epidermis (40).
2.5.2.2. Squamous Cell Carcinoma
It develops in the squamous cells of the outer skin layer (41).
2.5.2.3. Melanoma
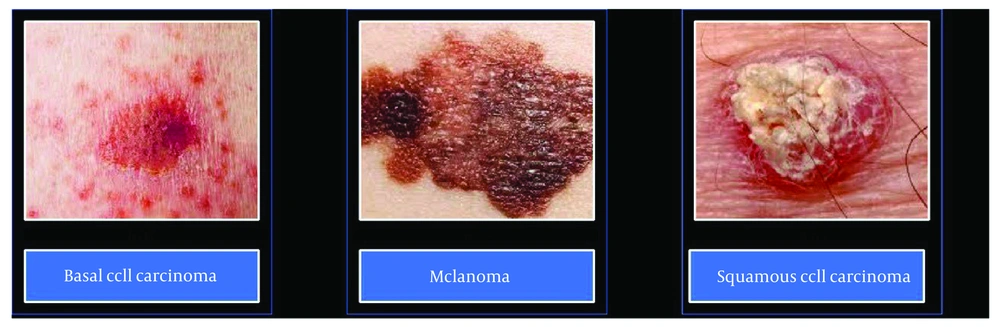
Melanoma, which develops from melanocytes, is the most aggressive form of skin cancer due to its potential to metastasize to other organs. Other rarer skin cancers include Kaposi Sarcoma, Merkel Cell Carcinoma, Sebaceous Gland Carcinoma, and Dermatofibrosarcoma Protuberans (42). Different types of skin cancers are represented in Figure 2.
2.5.3. Stages of Skin Cancer
Melanoma is staged from 0 to IV: Stage 0 (melanoma in situ) is confined to the epidermis; stage I is a low-risk melanoma treatable by surgery; stage II has a higher risk of recurrence but no metastasis; stage III involves spread to nearby lymph nodes; and stage IV indicates distant spread to lymph nodes and organs. Non-melanoma skin cancers, such as BCC and SCC, are staged based on tumor thickness, size, extent, and lymph node involvement, with stages ranging from 0 (in situ) to IV (invasive). Accurate staging is crucial for determining treatment and prognosis (43).
2.5.4. Prevention
Protecting the skin from UV rays through the use of shade, protective clothing, and broad-spectrum sunscreen helps prevent skin cancer. A plant-based diet rich in omega-3s, vitamins C and D, and antioxidants such as beta-carotene and lutein further supports prevention (44).
2.5.5. Treatment Options for Skin Cancer
Cryotherapy uses liquid nitrogen to freeze and remove cancer cells, while excisional surgery and Mohs micrographic surgery remove or target cancerous cells while preserving healthy tissue. Curettage and electrodesiccation are commonly used for BCC and SCC, scraping away cancer cells and destroying remnants with electric needles. Chemotherapy, immunotherapy, radiotherapy, and photodynamic therapy provide additional treatment options, targeting cancer cells through medications, radiation, or light-sensitive compounds (45).
3. Results
Current skincare trends are shifting towards natural, eco-conscious products featuring ingredients such as Aloe vera, turmeric, and tea tree oil for their anti-inflammatory and antibacterial benefits (46-50). There is also a growing interest in functional skincare that supports the skin's microbiome and incorporates adaptogens like ashwagandha to address stress-related skin issues. The emphasis on sustainability, "skinimalism," and traditional remedies like bakuchiol is driving the popularity of clean, effective skincare.
3.1. Herbal Remedies for Skin Care
Herbal therapy is gaining popularity due to its perceived lower side effects and holistic approach to treating skin conditions such as rashes and skin cancers. Herbs like chamomile and turmeric support long-term health, while allopathic treatments provide quick relief but may cause side effects, such as skin thinning. Herbal remedies are generally safer for long-term use, though they may cause allergies in some cases. In contrast, allopathic treatments often require monitoring due to potential side effects (51). Table 3 lists some commonly used herbs and their skincare benefits, while Figure 3 illustrates herbal marketed preparations.
| Drug | Botanical Name/Family | Active Constituent | Use | Reference |
|---|---|---|---|---|
| 1. Aroroba (Goa powder, Bahia powder) | Andira aroroba, Fabaceae | Chrysarobin, dithranol and chrysophanic acid | Ringworm, psoriasis, and eczema | (52, 53) |
| 2. Thorny Pigweed | Amaranthus spinosus, Amaranthaceae | Rutin, phenolic acid, ferulic acid, and linoleic acid | Eczema, sunburn, psoriasis | (54) |
| 3. Sarsaparilla | Smilax ornata, S. regeli, Smilacaceae | Sarsaponin, phytosterols, and flavonoids | Psoriasis, anti-inflammatory, acne, sun burns, anti-fungal | (55) |
| 4. Papaya | Carica papaya, Caricaceae | Papain, chymopapain, cystatin, α-tocopherol | Used in Face creams, cleansers, and psoriasis, wrinkle reduction | (56) |
| 5. Turmeric | Curcuma longa, Zingiberaceae | Curcuminoids, curcumin, des bis- methoxycurcuminoids | Anti-parasitic, dermatitis, psoriasis, Antimicrobial, fungicidal | (57) |
| 6. Gotu Kola (Brahmi) | Centella asiatica, Hydrocotyle asiatica, Apiaceae | D-limonene, α-terpineol, β-caryophyllene, α-pinene, eugenol | Anti-oxidant, anti-acne, antibacterial, anti-inflammatory and used in treatment of eczema and psoriasis | (58) |
| 7. Holy basil | Ocimum sanctum, Lamiaceae | β-caryophyllene, eugenol, vanillin, rosmarinic acid, ursolic acid, gallic acid and vanillic acid | Anti-inflammatory, antimicrobial activity | (59) |
| 8. Red Sandalwood | Pterocarpus santanlinus, Fabaceae | Santalin, santalol, santarubin, flavonoids, pterostilbenes, and lignans (savinin, calocedrin), | Post–acne and facial scars, cooling effect, improves complexion, reduce cutaneous inflammation and skin blemishes | (60) |
| 9. Garlic | Allium sativum, Liliaceae | Alliin, allicin, ajoenes and quercetin | Antifungal, anticarcinogenic, antioxidant, anti-inflammatory and promotes skin healing | (61) |
| 10. Pomegranate | Punica granatum, Punicaceae | Punicalagins and ellagitannin | Astringent with cooling properties | (62) |
| 11. Coriander | Coriandrum sativum, Apiaceae | Linalool, α-pinene, camphor, citronellal, geraniol, anethole | Antimicrobial, antioxidant, anti-inflammatory, anti-cancer and cooling effect | (63) |
| 12. Honey | Apis mellifera, Apidae | Enzymes, hydroxycinnamic acid, flavonoids, and organic acids, melittin | Astringent, antioxidant, bacteriostatic, anti-inflammatory and antimicrobial properties, used in wound healing and sunburns | (64) |
| 13. Sweet flag | Acorus calamus, Acoraceae | α, β, cis-asarone | Antibacterial, anti-fungal, anti-inflammatory, used in dusting powders, and skin lotions | (65) |
| 14. Camel thorn bush | Alhagi maurorum, Fabaceae | Flavonoids, fatty acids, coumarins, glycosides, steroids, vitamins, alkaloids, tannins, and triterpenes | Skin wounds, skin irritations, dermatitis and allergic reactions | (66) |
| 15. Aloe vera | Aloe barbadensis Miller, Liliaceae | Aloe-emodin, aloin, aloesin, emodin, vitamins A, C, and E and acemannan | Emollient, anti-inflammatory, antimicrobial, wound healing properties used in preparation of moisturizers and sunscreens, | (49) |
| 16. Galanga | Alpinia galanga, Zingiberaceae | Galangin, kaempferide, alpinin, zerumbone galangal acetate, and 1,8-cineole | Anti-inflammatory, used in dusting powders | (67) |
| 17. Oat | Avena sativa, Poaceae | Avenanthramide, ferulic acid, gentisic acid, vanillic acid, syringic acid | Anti-inflammatory, anti-oxidant and used in preparation of moisturizers, Skin tonics | (68) |
| 18. Neem | Azadirachta indica, Meliaceae | Azadirachtin, nimbolide, and gedunin, nimbidin, nimbin, quercetin, salannin, limonoids | Anti-oxidant, anti-inflammatory, antiseptic, antibacterial and reduce dark spots | (69) |
| 19. Marigold | Calendula ofjicinalis, Asteraceae | Lutein, zeaxanthine, esculetin, rutin, limonene, calendic acid | Anti-fungal, anti-inflammatory, antiseptic and wounds healing | (70) |
| 20. Ginseng | Panax ginseng, Araliaceae | Ginsenosides or panaxosides, peptides, polyacetylenic alcohols | Anti-aging, anti-inflammatory and wound healing | (71, 72) |
Recent dermatology research focuses on herbal nanoformulations to improve the delivery and efficacy of bioactive compounds for skin treatments. For example, turmeric's curcumin is encapsulated in nanoparticles to enhance its bioavailability and effectiveness in treating conditions such as acne, psoriasis, and eczema (73). Green tea extract, incorporated into lipid-based nanocarriers, has demonstrated enhanced antioxidant and UV-protective properties, aiding in the prevention of photoaging and skin cancer (74). Additionally, Aloe vera has been formulated into nanoemulsions, improving its wound-healing and hydrating properties, thereby providing faster relief for burns and other skin irritations (75). The use of nanoformulations in dermatology enables targeted therapy, reduced side effects, and enhanced stability of herbal compounds, positioning them as a promising area of research for the future of skincare treatments.
4. Conclusions
Skin diseases are often complex and require long-term management. While conventional treatments are effective, herbal medicines offer a complementary option with fewer side effects. Herbs with anti-inflammatory and antimicrobial properties can enhance skincare, particularly when combined with standard therapies. Ongoing research is essential to ensure the safe and effective use of these natural remedies, paving the way for more holistic and personalized skincare solutions. Herbal remedies provide a promising addition to conventional treatments, offering natural, gentle alternatives with potential benefits such as fewer side effects. As research advances, incorporating herbs into skincare routines could improve treatment outcomes and support overall skin health.