1. Context
Trichoscopy is a non-invasive bedside technique used to classify alopecia as either scarring or non-scarring, and to evaluate disease prognosis (1). It often reduces the need for skin biopsies by identifying key trichoscopic markers of activity and severity that help guide treatment choices, whether medical or surgical. Some markers indicate potential hair regrowth in scarring alopecia, while others signal end-stage scarring. Trichoscopy also assists in selecting biopsy sites, particularly in cases where diagnosis is challenging, and helps distinguish between clinically similar alopecia types that require different treatments.
2. Objectives
This article will explore the trichoscopic patterns of both non-scarring and scarring alopecia.
3. Methods
In this review article, we have conducted extensive research using databases and search engines such as Medline, PubMed, Embase, Scopus, Cochrane Library, and Science Direct. The keywords included “alopecia,” “cicatricial,” “non-cicatricial,” “trichoscopy,” and “dermoscopy,” either singly or combined. Various studies, case reports, original articles, prior review articles, as well as personal experiences from our clinical practice, have been considered while writing this narrative review.
4. Results
4.1. Nonscarring (Non-cicatricial) Alopecia
4.1.1. Androgenetic Alopecia
The trichoscopic patterns differ at every stage or grade of androgenetic alopecia (AGA). The Norwood-Hamilton classification describes seven grades of AGA in males. In females, the Sinclair classification describes three grades of AGA in females (2). The trichosopic features of non-scarring alopecia are summarised as follows in Table 1 (1, 2).
| Types of Nonscarring Alopecia | Trichoscopic Hallmarks |
|---|---|
| AGA | Hair diameter variability, yellow dots, vellus hair, perifollicular pigmentation |
| AA | Exclamation mark hair, vellus hair, yellow dots, coudability sign, black dots |
| Tinea capitis | Comma hair, cockscrew hair, morse code hair, zigzag hair, bent hair, block hair, i – hair |
| Tractional alopecia | Decreased hair density, broken hair, tulip hair |
| TTM | Irregularly broken hair, coiled hair, v sign, hook hair, flame hair, tulip hair, hemorrhages, black dots, hook hair, yellow dots |
| AA incognita | Empty yellow dots, small hair in regrowth, yellow dots with vellus hair, pigtail hair |
Abbreviations: AGA, androgenetic alopecia; AA, alopecia areata; TTM, trichotillomania
In AGA, trichoscopy needs to be performed in four quadrants of the scalp: Frontal, parietal, vertex, and the occiput (3). In male pattern hair loss, trichoscopic evaluation aids in grading and assessing the severity of AGA. It is a valuable bedside tool to assess the eligibility of the patient for hair transplant surgery by evaluating the donor area. The key points to evaluate for hair transplant surgery include scalp laxity, density of follicular grafts, and the interfollicular area in the donor region. In the recipient area, trichoscopy is essential to identify any secondary actinic changes such as atrophy, scars, seborrheic dermatitis, or other inflammatory scalp conditions that may impact the surgical outcome.
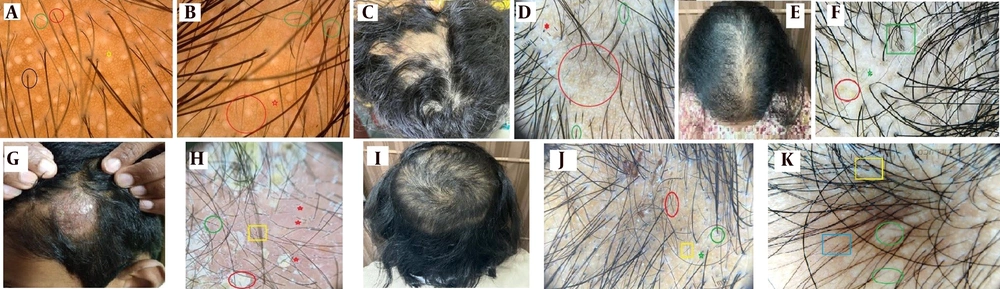
In female pattern hair loss (FPHL), trichoscopic assessment (Figure 1A) is crucial to differentiate between chronic telogen effluvium and FPHL or their concomitant coexistence. It is common to encounter chronic telogen effluvium and FPHL simultaneously. Common causes of FPHL include polycystic ovarian syndrome, hypothyroidism, premenopause or menopause, ovarian tumors, systemic corticosteroids, and adrenal tumors (4).
Non scarring alopecia; A, female pattern hair loss (FPHL) trichoscopy- single hair follicular unit (black circle), anisotrichosis (red and green circles), follicular white dots (yellow star); B, androgenetic alopecia (AGA)- miniaturised hair (red circle), peripilar sign (green circles), empty follicular white dots (red star); C, alopecia areata (AA)- smooth reticular patches on vertex; D, AA-Trichoscopyblack dots (red star), exclamation mark hair (green circles) and regrowing vellus hair (red circle); E, AA incognita- widening of partition mimicking FPHL; F, AA incognita dermoscopy- yellow dots (red circle), coudability hair (green square) and pigtail vellus hair (green star); G, inflammatory tinea capitis; H, tinea capitis dermoscopybroken hair (red-stars), morse code hair (green circle), zigzag hair (yellow-square), whitish-yellow scaling (red circle); I, TTM with Friar tuck sign; J, TTM trichosopy-hair breaks at variable lengths (red circle),black dots (green star), perifollicular scaling (green circle) and vellus hair (yellow square); K, trichoscopy- traction alopeciawhitish structureless areas (green circles), hair casts (yellow square), vellus hair (blue square).
In cases of telogen effluvium, the underlying etiology is most commonly nutritional deficiencies, such as iron deficiency anemia, vitamin B12 and vitamin D deficiencies, and psychosocial stress. A detailed clinical history of past and present comorbid conditions helps direct an appropriate diagnosis of telogen effluvium. In tropical countries like India, recent or past infectious illnesses such as dengue fever, chikungunya fever, COVID-19 infection, and scarlet fever have topped the list of etiologies. However, any other illness could also lead to telogen effluvium. Appropriate investigation and correction of these underlying etiological parameters can result in successful treatment.
On the other hand, FPHL may require surgical intervention in advanced or end-stage forms. Weight reduction and exercise routines are important aspects of treatment for FPHL. Some patients may benefit from antiandrogens such as finasteride or dutasteride, spironolactone, cyproterone acetate, and flutamide for FPHL management, following appropriate investigations.
Prominent signs in diffuse androgenetic alopecia (DUPA) include heterogeneity of hair thickness, globally reduced hair density, perifollicular discoloration (peripilar sign), increased vellus hair, and a large number of follicular units with only one emerging hair shaft. Trichoscopy of typical patterned AGA (Figure 1B) reveals similar features in the parietotemporal areas of the scalp (4).
Trichoscopy of chronic telogen effluvium reveals yellow dots, some coiled hair, a honeycomb pigment network, empty follicular openings, short regrowing vellus hair, and globally reduced hair density. It is a diagnosis of exclusion when the trichoscopy does not demonstrate the pathognomonic signs of other types of alopecia (5).
4.2. Alopecia Areata
Alopecia areata (AA) is an autoimmune hair disorder characterized by focal or multiple patches of hair loss (Figure 1C). While it commonly involves scalp hair, it can also affect hair on other body parts. Alopecia Areata exhibits a variety of trichoscopic signs depending on the stage, severity, and type of the condition.
The classical Trichoscopic picture of AA includes exclamation mark hair, small vellus hair, black dots, yellow dots, circle hair, and tapering hair (Figure 1D). The coudability sign is indicative of disease activity and progression.
In severe variants like Alopecia Universalis and Alopecia Totalis, Trichoscopy reveals vellus hair in very few areas, along with prominent yellow dots. Yellow dots represent distended follicular infundibula filled with sebum and keratin remnants. Black dots are remnants of broken hair shafts within the follicular ostia.
An increased number of yellow dots and black dots signifies greater severity and activity of AA (6).
4.3. Alopecia Areata Incognita
Alopecia Areata Incognita, also known as diffuse AA, is a rare form of AA commonly described in middle-aged women. It was first described by Rebora in 1987 (7). Clinically, it is characterized by a sudden increase in hair loss occurring within weeks, and in these patients, the hair pull test is positive. In this variety, the typical patchy hair loss is absent, but there is abrupt and intense hair loss.
Clinically, it closely resembles chronic telogen effluvium, FPHL, or diffuse unpatterned alopecia (DUPA) (Figure 1E). In such cases, trichoscopy plays a crucial role in confirming the diagnosis. Specific features such as yellow dots, black dots, circle hair, exclamation mark hair, and vellus hair are present (Figure 1F).
Sometimes, premenopausal women may also show a trichoscopic picture resembling patterned or diffuse AGA, including hair diameter variability, thinning, and reduced hair density, along with features of AA. Trichoscopy in these cases helps guide the clinician to initiate appropriate immunosuppressive therapy for AA incognita.
In our case, the patient presented with FPHL in the frontotemporal area along with features of AA in the occipital and parietal regions, which were diagnosed through trichoscopy and histopathology.
4.4. Tinea Capitis
Tinea Capitis can present in inflammatory forms (Kerion and Favus) (Figure 1G) and non-inflammatory forms (Grey Patch and Black Dot). Trichoscopy is nearly 97% sensitive in diagnosing tinea capitis. Characteristic trichoscopic features include broken hair, comma hair, black dots, corkscrew hair, zigzag hair, and perifollicular scaling (Figure 1H), which are highly pathognomonic for tinea capitis (8).
The closest differentials for non-inflammatory variants include AA and trichotillomania (TTM), while for inflammatory variants, pyodermas top the list of differentials.
Tinea capitis is common in children, and trichoscopy serves as the best non-invasive confirmatory test for diagnosing Tinea Capitis. It eliminates the need for skin biopsy in children.
4.5. Trichotillomania
Trichotillomania is a psychological and habitual hair disorder characterized by repetitive and recurrent hair pulling, resulting in well-circumscribed hair loss (Figure 1I). It predominantly affects teenagers, especially females. The closest differentials, both clinically and in Trichoscopy, are AA and tinea capitis.
Unique trichoscopic characteristics of TTM include frayed hair, variable hair length, coiled hair, pigtail hair, black dots, hemorrhages, and trichoptilosis (split hair) (Figure 1J). Notably, it lacks exclamation mark hair and yellow dots, which are pathognomonic of AA (9).
Fluoxetine at optimal dosing combined with psychological counseling is the treatment of choice for this condition. While the scalp is the most commonly affected site, other body hair may be involved in rare cases. Severe cases may present with a tonsure pattern of hair loss over the crown area of the scalp.
The diagnosis can be confirmed through clinical history and trichoscopy. A skin biopsy is not always necessary, as trichoscopic features provide sufficient guidance for initiating treatment in patients with this disorder.
4.6. Tractional Alopecia
Tractional Alopecia is common in the Indian population, particularly among Sikhs and school-going girls, where hair is tied too tightly. Chronic pulling force on the hair can lead to permanent hair loss; however, with early intervention, hair regrowth can occur within months. Trichoscopy is highly beneficial for diagnosing this condition.
The closest differentials include TTM, Patterned Hair Loss, and AA. Trichoscopy reveals pathognomonic signs (Figure 1K), such as frayed hair, trichomalacia (soft, fragile, and broken hair), reduced follicular openings, decreased hair density, freely mobile hair casts at the periphery of the affected hair loss zone, a honeycomb pattern, regularly distributed white dots, and many miniaturized hairs (10).
Tractional Alopecia commonly affects the hairline in the temporal and frontal areas, mimicking Patterned Alopecia. However, it can also involve the occipital area in patients who tie their hair into ponytails or judas on a daily basis.
4.7. Scarring (Cicatricial) Alopecia
4.7.1. Discoid Lupus Erythematosus
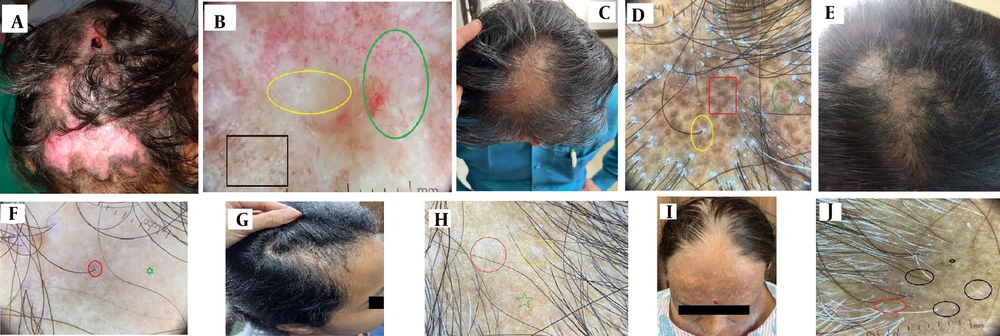
Discoid lupus erythematosus (DLE) is the most frequent type of lupus leading to scarring alopecia, although it is uncommon on the scalp (Figure 2A) (1). The inflammatory process in DLE manifests trichoscopic signs of active disease, such as erythema and scaling, which affect both perifollicular and interfollicular areas (11). Table 2 trichoscopic hallmarks of various scarring alopecias (12).
Scarring Alopecia; A, discoid lupus erythematosus (DLE) of scalp with cicatricial alopecia; B, DLE dermoscopy- telangiectasias (green circle), whitish structureless area (yellow-circle), peripheral pigment peppering (Black square); C, lichen planopilaris (LPP)-scarring frontovertical alopecia with few spared intervening hairs; D, LPP dermoscopy-peripilar sheathing and scaling (yellow-circle), dark brown globules (red square), blue-grey globules (green circle) (target sign); E, PPB with foot prints in snow appearance; F, PPB dermoscopy white structureless area (green star), lone terminal hair from single follicular unit (red circle); G, morphea- en-coup de sabre cicatricial alopecia; H, morphea dermoscopy- white structureless area (red circle), fibrotic beam (yellow square), absent follicular ostia (green star); I, frontal fibrosing alopecia (FFA)- progressive cicatricial fronto-temporal alopecia; J, FFA- dermoscopy- lone terminal hair (black circles) with loss of vellus hair, There is perifollicular scaling (red circle), scarred structureless area (black star).
| Variables | Definition |
|---|---|
| DLE | (1) Absence of follicular openings; (2) abnormal vascular patterns (giant capillaries, thick arborizing vessels); (3) coarse perifollicular hyperkeratosis |
| LPP | (1) Absent follicular ostia; (2) hyperkeratosis and perifollicular erythema; (3) preserved white pinpoint dots in dark skin; (4) mottled blue-gray dots; (5) tubular scaling with minimal interfollicular scaling; (6) plume-like hairs (2 - 4/follicle) |
| Pseudopelade of Brocq | Non-specific patterns of porcelain whitish structureless areas with complete loss of follicular ostia and few dystrophic hairs at periphery |
| Morphea | (1) Fibrotic beams; (2) linear arborizing vessels (lilac ring); (3) pin point white dots; (4) loss of follicular openings; (5) thick telangiectasia (dilated subpapillary plexus); (6) pili torti, broken hair |
| FFA | (1) Absence of follicular ostia; (2) concentric perifollicular hyperkeratosis and erythema; (3) absence of vellus hair; (4) presence of lonely hairs or fragile hairs (pili torti) |
Abbreviations: DLE, Discoid lupus erythematosus; LPP, lichen planopilaris; FFA, frontal fibrosing alopecia.
Ongoing inflammation in DLE disrupts the honeycomb pigment network and results in pigment incontinence, culminating in interfollicular speckled or mottled dark brown to blue-gray dots (in skin of color) on trichoscopy (13, 14). This contrasts with lichen planopilaris (LPP), where such dots are predominantly perifollicular (13, 15-17). Another distinguishing feature of DLE is that it affects all follicles uniformly, whereas LPP often spares some intervening follicles (1).
Hyperkeratotic perifollicular scales, an indicator of inflammatory changes, are common to both DLE and LPP. Other trichoscopic features of DLE include absent follicular ostia, prominent keratotic follicular plugs (giant yellow dots at the plaque periphery), and various vascular patterns such as giant capillaries, thick arborizing vessels, and telangiectasia (Figure 2B).
The presence of follicular red dots indicates viable follicles and the potential for hair regrowth with prompt treatment (18-20). In late-stage disease, trichoscopy shows skin atrophy, milky-red areas, and loss of follicular openings. A combination of dilated, tortuous vessels and keratotic plugs is highly specific for chronic DLE (21).
Recently described trichoscopic traits of DLE include the blue-white veil (resulting from pigment incontinence) and white rosettes (indicative of periadnexal keratin retention) (19).
4.7.2. Lichen Planopilaris
Lichen planopilaris is the most common lymphocytic scarring alopecia (Figure 2C), selectively targeting hair follicles while relatively sparing interfollicular areas (15, 16, 22). Trichoscopic signs of folliculocentric inflammation (Figure 2D) include perifollicular erythema and peripilar casts, which represent tubular scales typically seen at the periphery of lesions. Blue-gray dots in a target pattern, particularly in skin of color, signify perifollicular pigment incontinence (11, 23, 24).
In longstanding untreated cases, trichoscopy reveals absent follicular openings and white dots, indicative of follicular fibrosis (13, 15, 16). A notable feature described by some authors is a tuft of hair containing 2–4 shafts ensheathed by scaling (25).
In cases of diagnostic uncertainty, perifollicular erythema and hyperkeratosis, which indicate the active phase of LPP, serve as crucial guiding signs for selecting biopsy sites to increase diagnostic yield (26). Interfollicular skin typically shows relative sparing with a honeycomb pigment pattern and a few remaining terminal hairs. Acquired pili torti, a feature commonly observed in primary cicatricial alopecias, is also seen in LPP (27).
4.7.3. Pseudopelade of Brocq
Pseudopelade of Brocq is both a clinical and histological diagnosis of exclusion. It represents the end stage of many cicatricial alopecias and lacks active inflammation. The scarring pattern and loss of follicular ostia create a characteristic "footprints in the snow" appearance (Figure 2E).
trichoscopic features are non-specific and include scarred hypopigmented areas with follicular paucity (Figure 2F) and a few dystrophic hairs (1, 28, 29).
4.7.4. Morphea
The trichoscopic features of Morphea vary according to the disease stage (Figure 2G). Key findings include fibrotic beams (Figure 2H) and small whitish patches traversed by linear arborizing vessels, sometimes exhibiting a lilac ring (30). These features align with histological findings of dermal sclerosis (31).
Perifollicular fibrosis can distort hair shafts, causing pili torti, which easily break to form black dots or broken hairs. As the sclerotic process progresses, trichoscopy reveals the absence of follicular ostia and pinpoint white dots (32).
A recently described trichoscopic feature of thick telangiectatic vessels corresponds to an ectatic subpapillary plexus. This vascular pattern mimics those seen in Discoid Lupus and Dermatomyositis. Such findings may result from prominent scalp vascularization, more visible in early inflammatory Morphea.
Additional findings include peripilar casts in inflammatory Morphea and short regrowing hairs in long-standing disease (32).
4.7.5. Frontal Fibrosing Alopecia
In Frontal fibrosing alopecia (FFA) (Figure 2I), inflammatory manifestations are more subtle (11). The trichoscopic hallmark (Figure 2J) is the loss of vellus hair along the hairline (33).
Indicators of disease activity include concentric perifollicular erythema and hyperkeratosis, while indicators of disease progression include the loss of vellus hair and absence of follicular ostia. These features help distinguish FFA from LPP and TTM.
The Pseudo-Fringe Sign (described by Pirmez et al.) refers to retained lonely terminal hairs (loss of vellus follicles) in the frontotemporal region. This contrasts with the True Fringe Sign in Traction Alopecia and TTM, where vellus hair are retained along the frontotemporal marginal hairline (34).
Less frequent trichoscopic findings in FFA include broken hair and fragile hair. Trichoscopic evaluation of eyebrows reveals dystrophic hair, regrowing hair, yellow dots, loss of follicular ostia, and diffuse erythema. These features help distinguish FFA from AA affecting the eyebrows in its early stages (35).
In men, FFA-related loss of follicular density along the frontotemporal hairline may mimic AGA. The presence of perifollicular erythema, hyperkeratosis, hair diameter variability, miniaturization, vellus hair, reduced terminal hair per follicular unit, and areas of follicular scarring distinguish FFA from AGA (36).
4.8. Advantages and Limitations of Trichoscopy in the Diagnosis of Alopecia
Trichoscopy's utility lies in its bedside accessibility and user-friendly design. Its non-invasive nature has contributed to its growing popularity among dermatologists. Key advantages include its ability to evaluate the activity and severity of alopecia, assist in narrowing differential diagnoses, monitor treatment responses directly at the bedside, and guide biopsy site selection for histopathological analysis (37). However, trichoscopy has certain limitations, such as a steep learning curve, variability between devices and observers, potential artifacts that can obscure findings, the absence of standardized trichoscopic nomograms, and differences in observations across various Fitzpatrick skin types (37).
Histopathology, in comparison with trichoscopy, has its merits and demerits in the diagnosis of alopecias. It provides definitive data by identifying specific hair follicle stages (catagen or telogen) and structural changes, enabling early and precise diagnosis of hair cycle disorders and thus serves as the diagnostic gold standard (38). It is recognized for its ability to confirm clinical and trichoscopic findings. On the contrary, the utility of histopathology is limited by its invasive nature and reliance on specialized expertise. A combined clinical, trichoscopic, and histologic approach may provide the most comprehensive evaluation for alopecia cases (38).
There are multiple descriptive studies in the Western literature about trichoscopic patterns in alopecias; however, in the Indian context, such studies are meagre, and there exist a few practice and research gaps. Barriers to the routine use of trichoscopy include a lack of awareness among dermatologists about its value for examining the hair and scalp, limited training resources, and the cost of equipment (39). As a relatively new practice, many dermatologists are unfamiliar with trichoscopic patterns and do not have access to specialized training. Additionally, scalp dermoscopy is often not covered in dermatology conferences, making it harder to integrate this technique into everyday practice. To narrow the gap, it is imperative to integrate trichoscopy training into Continuing Medical Education (CME) through workshops, conferences, and online modules (39).
5. Conclusions
Trichoscopy is a good bedside tool to narrow down the differentials of hair loss. An updated and ongoing knowledge of trichoscopic signs in respective types of alopecia is necessary for prompt diagnosis, classification, and treatment.