1. Context
Acne is a multifactorial inflammatory condition affecting a significant portion of the global population, ranking among the top three most prevalent dermatological conditions worldwide. The condition typically begins during puberty, with the highest prevalence observed in adolescents aged 14 to 17 years. While acne tends to appear earlier in females, it is often more severe in males due to hormonal fluctuations (1). It is caused by multiple pathogenic factors, including sebaceous hypersecretion, characterized by excessive sebum production; follicular hyperkeratinization, which, along with alterations in the skin microbiota, triggers the inflammatory process and dysfunctions in both innate and adaptive immunity. This leads to the formation of open and closed comedones, in addition to inflammatory processes that can affect the pilosebaceous units throughout the body (2).
Acne can present in varying degrees of severity, classified from grade 0 to grade 5, depending on the extent of the lesions, systemic involvement, and overall impact on the individual. This dermatological condition encompasses both non-inflammatory and inflammatory lesions, which are often associated with varying degrees of scarring. Grade 1 acne primarily involves non-inflammatory lesions, known as comedones (commonly referred to as blackheads). Grade 2 acne is characterized by blackheads and small inflamed lesions, including yellow pus-filled pustules. Grade 3 acne presents with blackheads, small lesions, and larger, deeper, painful, reddish, and well-inflamed lesions, commonly referred to as cysts. Grade 4 acne involves blackheads, small lesions, large cystic formations, multiple interconnected abscesses, and irregular scarring, which can lead to significant deformities in the affected areas. Grade 5 acne, also referred to as acne fulminans, is a rare and severe form of the condition that includes extensive lesions alongside systemic symptoms such as fever, joint pain, and malaise. This grade is more common in men and typically affects the chest, back, and face (3).
Acne, as a chronic condition, does not have a definitive cure; however, it can be effectively managed. Treatment options include conventional topical, systemic, and hormonal therapies, with the choice of approach depending on the mode of manifestation, the severity of the condition, and the individual needs of each patient (4). These factors must be carefully assessed, as each grade of acne has distinct clinical implications and requires a tailored treatment strategy. For instance, lower grades (1 and 2) are typically treated with topical therapies, including retinoids and antimicrobial agents. Grades 3 and 4 often require systemic treatments, such as oral antibiotics or isotretinoin. Grade 5 acne, the most severe form, usually demands a combination of systemic therapies and close medical supervision to address both the dermatological and systemic symptoms (5).
In addition to traditional treatments, recent research has explored the use of cannabidiol (CBD) as an alternative for treating various skin pathologies, including acne. According to the literature, CBD appears to interact with the cutaneous endocannabinoid system (ECS), primarily targeting CB1 and CB2 receptors present in various skin structures, such as the epidermis and hair follicles. Furthermore, CBD has been shown to suppress sebocyte proliferation and exert anti-inflammatory effects. Despite its promising therapeutic potential, the use of CBD in pharmaceutical products has faced stigma and regulatory challenges in some regions. Due to the relevance of the topic, this review aims to provide a comprehensive overview of these findings, while also addressing the legislation surrounding the approval of CBD for use in cosmetics across different countries.
2. Evidence Acquisition
A systematic literature search was conducted in the Web of Science and PubMed databases using the following specific keywords: "Cannabidiol", "CBD", "acne vulgaris", "endocannabinoids", "skin", "topical application", and "dermatology". The search strategy included studies published up to 2024.
3. Results
3.1. Acne-inducing Factors
During puberty, a series of biological changes occur in the body due to the significant release of hormones. The increased production of androgens stimulates hypersecretion of sebaceous glands, hyperkeratinization of the follicular ostium, and alterations in the composition of sebum, all contributing to the pro-inflammatory events characteristic of acne vulgaris (1). Androgen receptors are found in the sebaceous glands and the outer root sheath of the hair follicle. These cellular structures respond to the action of testosterone and dihydrotestosterone (DHT), which stimulate sebum production. Through androgenic action, testosterone is converted into DHT by the enzyme 5α-reductase. Dihydrotestosterone then stimulates the sebaceous glands to synthesize fatty acids and triglycerides, leading to increased sebum excretion. The accumulation of free fatty acids in the glandular infundibulum over time is believed to irritate the surrounding epithelium, triggering hyperkeratinization (the initial stage of comedogenesis) and eventually inflammation, thereby contributing to the development of acne (2).
Another contributing factor to the development of acne is the alteration of the skin microbiota. The bacterium Cutibacterium acnes plays a crucial role in maintaining skin homeostasis (6). However, the colonization of C. acnes in the pilosebaceous region — particularly in areas densely populated with sebaceous follicles that provide a lipid-rich environment — represents a pathological feature of acne. In this environment, C. acnes triggers the formation of comedones and the release of inflammatory mediators. It initiates an inflammatory response, leading to the migration of inflammatory cells, such as lymphocytes and neutrophils, which release inflammatory substances like reactive oxygen species (ROS) and tumor necrosis factor-alpha (TNF-α). Consequently, these inflammatory mediators exacerbate inflammation and cause chemical damage to the follicular epithelium and proximal dermis (7).
3.2. Conventional Treatment
In milder cases, the use of cleansing soaps, lotions, or topical gels is recommended. Mild and non-extensive clinical presentations are typically treated with topical medications such as benzoyl peroxide, azelaic acid, dapsone, adapalene, tretinoin, retinoids, or even antibiotics. However, for more severe cases of acne, additional treatments are employed, including topical and oral antibiotics, as well as hormone-based therapies. Among these, oral isotretinoin, a retinoid, is particularly notable for its effectiveness and is recommended when other treatment options fail to improve the severity of acne (8). Although isotretinoin is typically regarded as safe and well-tolerated, it can cause significant adverse effects, such as an increased risk of depression during use. Additionally, women must avoid pregnancy due to its teratogenic potential. These side effects may discourage patients from starting or continuing isotretinoin therapy, emphasizing the urgent need for safer and equally effective therapeutic alternatives (7). Research in this area aims to identify dermatological actives capable of addressing multiple stages of acne pathophysiology, such as excessive sebum production, sebocyte proliferation, and inflammation, while avoiding any side effects during treatment (9).
3.3. Alternative Treatment
Recently, numerous companies have invested in plant-based ingredients, driven by increasing concerns over environmental sustainability and the search for innovative ingredients. As a result, products derived from Cannabis sativa have gained significant attention, showing rapid growth and diversification within the skincare sector. However, confusion remains regarding the benefits of these products, largely due to uncertainties surrounding their composition (10). Presently, CBD constitutes up to 40% of the compounds found in C. sativa. When isolated, CBD lacks the psychoactive effects associated with the plant, positioning it as a promising option for treating skin conditions, particularly inflammation and acne, due to its antioxidant and anti-inflammatory properties (11). The identification of novel biological and pharmacological actions of topical cannabinoids has expanded therapeutic opportunities in skincare, given their ability to modulate inflammatory processes, including acne (12).
3.4. Endocannabinoid Skin System
In 1964, a study conducted in Israel by Mechoulam and Gaoni (as cited by Crocq) successfully isolated the chemical structure of Δ9-tetrahydrocannabinol (Δ9-THC), which later inspired Devane to discover cannabinoid receptors, identified as G-protein-coupled receptors (GPCR) CB1 and CB2. This discovery led to the identification of a new receptor system, known as the ECS (13). Humans possess an ECS that regulates various physiological processes, consisting of receptors, endocannabinoid molecules, and proteins responsible for their synthesis, transport, and degradation. Modulating the ECS presents a promising therapeutic target for a range of diseases, including dermatological conditions, as it plays a key role in maintaining skin homeostasis (14).
The ECS consists of a class of GPCR, specifically CB1 and CB2, which belong to the class A GPCR subgroup. These receptors can bind to endogenous lipid-derived ligands, such as anandamide (AEA) and 2-arachidonoyl glycerol (2-AG). They are distributed across various cell types, including human keratinocytes, melanocytes, dermal fibroblasts, and myoepithelial cells. Additionally, endocannabinoids exhibit activity at other receptor sites, such as transient receptor potential vanilloid 1 (TRPV1) and peroxisome proliferator-activated receptors (PPAR) (15, 16).
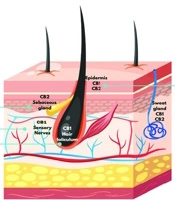
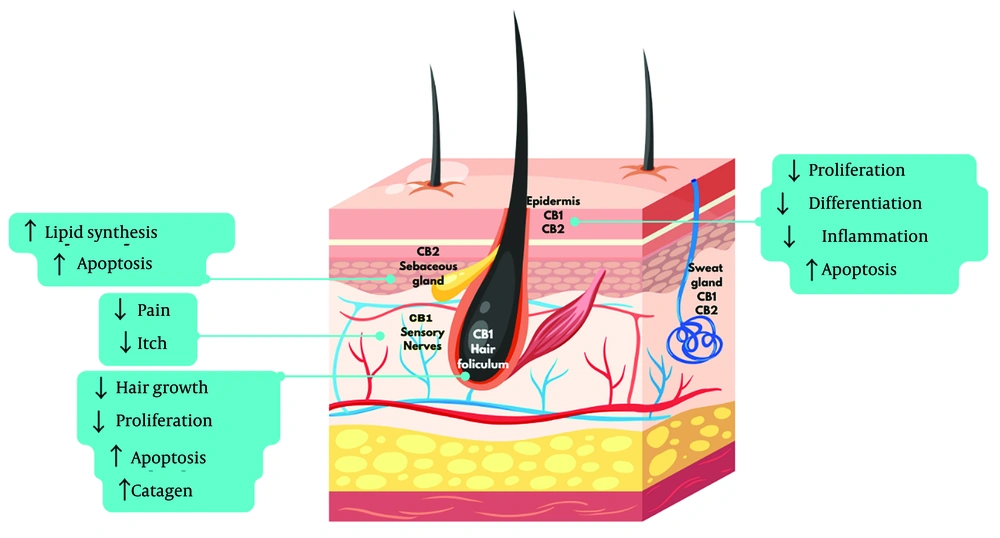
In the skin, CB1 and CB2 receptors are expressed in various structures, including the epidermis, sebaceous glands, and hair follicles. Their regulation plays a key role in inflammatory processes, cell proliferation, and sebum production. For effective binding to CB1 or CB2 receptors, ligands must possess high lipophilicity (15). In the skin (Figure 1), CB1 receptors are predominantly located in the stratum spinosum and granulosum of the epidermis, while CB2 receptors are found in the stratum basale, sebaceous glands, and hair follicle cells (17, 18).
Anandamide and 2-AG act as agonists for both CB1 and CB2 receptors, though peripheral tissue levels of 2-AG are higher compared to AEA. After release, endocannabinoids undergo neuronal reuptake and are quickly metabolized into inactive compounds by the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MGL) (19). While the signaling of the ECS can influence various aspects of skin biology, its dysregulation may contribute to the development of several skin pathologies, including acne. This system interacts with hair follicles, the epidermis, and sebaceous glands, playing a crucial role in maintaining normal skin physiology (20). The ECS works to inhibit the release of inflammatory mediators involved in both wound healing and the inflammatory processes that occur in the skin (21).
3.4.1. Cannabinoids
Cannabinoids are a group of bioactive compounds classified into three categories: Endocannabinoids produced by the human body, phytocannabinoids primarily derived from C. sativa (commonly known as marijuana or hemp), and synthetic cannabinoids that are artificially synthesized (22). Cannabis sativa produces over 100 phytocannabinoid compounds; however, only a select few are relatively abundant and active within the ECS (23). All of these compounds are classified as cannabinoids due to their structural similarity to endocannabinoids; however, most either do not bind to known cannabinoid receptors or do so with low efficiency. The most prevalent cannabinoids in C. sativa are Δ9-THC, CBD, and cannabinol (CBN) (24).
Most synthetic cannabinoids have been developed to investigate the function of the ECS while circumventing the restrictions associated with the use of phytocannabinoids. Examples of synthetic cannabinoids include dronabinol and its analogues, nabilone and rimonabant, which are utilized to treat conditions such as pain, loss of appetite, and obesity (25, 26). Cannabinoids exhibit both antagonistic and agonistic effects on the ECS, influencing functions such as keratinocyte proliferation, sebum production, hair growth, and inflammation. In dermatological pathophysiology, both activation and inhibition of CB1 and CB2 receptors are commonly observed. In the context of acne, inhibiting CB2 receptors reduces the production of basal lipids, suggesting that CB2 antagonists may be effective in treating skin conditions associated with sebaceous gland dysfunction (27).
3.4.2. Cannabidiol
Cannabidiol is the primary non-psychoactive phytocannabinoid found in the C. sativa plant. Unlike Δ9- THC, CBD does not alter the perception of reality. It is notable for its analgesic, anti-inflammatory, antioxidant, anxiolytic, antidepressant, neuroprotective, anticonvulsant, and anti-nausea properties. Additionally, CBD modulates the effects of Δ9-THC, helping to reduce undesirable effects associated with this molecule, such as anxiety, depression, and hallucinations (28).
Research on CBD has highlighted its potential in managing inflammatory and immune-related conditions, as it seems to act on key inflammatory pathways and receptors, thereby lowering cytokine production. This compound has gained attention for its innovative application in treating skin inflammation, including acne, due to its strong antioxidant and anti-inflammatory effects. Furthermore, CBD’s interaction with the ECS makes it a promising approach for addressing a range of skin disorders (12).
The CBD is a lipophilic molecule with a high partition coefficient (LogP 6.43), which accounts for its low bioavailability (13 - 19%) following oral administration. This limited bioavailability is primarily attributed to its poor water solubility in gastrointestinal fluids and significant first-pass metabolism. In contrast, cutaneous administration of CBD offers significant pharmacokinetic advantages. Due to its high LogP (> 3), CBD naturally tends to accumulate in the outermost layer of the skin, the stratum corneum (SC), forming a depot with minimal percutaneous absorption. However, its penetration is highly dependent on the formulation of the vehicle, which can either enhance or hinder absorption. The low molecular weight of 314.46 g/mol may facilitate its penetration (28).
Casiraghi et al. demonstrated that vehicles such as liquid paraffin, 80% propylene glycol, or a hydrophilic gel achieved the best permeation results (~20 μg/cm2 cumulative permeated amount at 24 h), whereas virgin olive oil, 80% polyethylene glycol (PEG) 400, hydrophobic ointment, or (trans) dermal patch showed the poorest performance (0.31 - 7.23 μg/cm2 cumulative permeated amount at 24 h) (12). Additionally, compared to inhaled pharmaceutical forms, topical CBD applications are associated with fewer respiratory side effects (29).
In vitro tests demonstrate that CBD acts on sebocytes by inhibiting lipogenesis and neutralizing acne-inducing agents such as arachidonic acid and testosterone. Additionally, CBD suppresses sebocyte proliferation without causing cytotoxicity and inhibits TNF-α expression induced by TLR2 and TLR4 agonists (15). In cases of skin inflammation, CBD interacts with the ECS primarily through the CB2 receptor, acting as an agonist of cannabinoid receptors coupled to inhibitory G proteins. Cannabinoid receptors are known to co-localize with TRPV1 channels, and CBD also functions as a TRPV1 agonist. During inflammatory processes, TRPV1 expression increases, leading to the release of pro-inflammatory cytokines and chemokines such as IL-1α, IL-6, and TNF-α, along with COX-2 (cyclooxygenase-2) and heightened leukocyte trafficking in the joints. The activation of TRPV1 ion channels results in the peripheral release of inflammatory neuropeptides, which contribute to neurogenic inflammation and further increase leukocyte trafficking. Consequently, the observed anti-inflammatory effects of CBD may be linked to the desensitization of TRPV1 ion channels, reducing inflammation and exerting an anti-rolling effect. Thus, the desensitization of TRPV1 by CBD could potentially halt the progression of inflammation (30).
However, some authors, such as Oláh et al. (as cited by Ferreira et al.), suggest that the lipostatic and antiproliferative effects of CBD are linked to the activation of TRPV4 ion channels rather than TRPV1 and TRPV2. Therefore, the lipostatic effects of CBD and the resulting Ca²⁺ influx would not be influenced by specific TRPV1 antagonists. These electrophysiological findings indicate that TRPV4 plays a key role in mediating the effects of CBD (18).
The activation of PPARs, particularly the PPARα and PPARγ isoforms, by cannabinoids is associated with their antiproliferative and anti-inflammatory effects. PPARγ is expressed in various cell types, including fibroblasts, keratinocytes, melanocytes, and sebocytes (15, 16).
3.5. Cannabidiol-Related Pre-clinical and Clinical Trials
Clinical trials evaluating the effectiveness of CBD in treating acne predominantly focus on topical and oral dosage forms. Currently, three clinical trials are registered on the National Library of Medicine platform (31). One phase 2 trial investigates the use of a 5% CBD topical formulation for acne (BTX1503), though the results are yet to be published (32). Another phase 1 study aims to evaluate the safety and efficacy of microneedling combined with CBD and hempseed oil for treating moderate to severe acne (33). The third trial examines the effects of a topical preparation containing CBD and hemp oil on erythema, skin appearance, and sebum production (34).
In addition to clinical trials, researchers have conducted in vitro and in vivo studies to explore and assess the clinical potential of CBD for acne. A summary of the main findings from these studies is presented in Table 1.
| Number | Study Type | Formulation | Protocol | Results | Ref. |
|---|---|---|---|---|---|
| 1 | In vitro | CBD solution at 1 and 10 μM concentration | - Single application on immortalized human sebocytes; - sustained treatment with 10 μM CBD over 14 days; - single dose of 50 μM CBD | -Reduced sebocyte proliferation, cell count, and lipogenesis; - a 50 μM single dose induced apoptosis-related cytotoxicity; - lipostatic, antiproliferative, and anti-inflammatory effects demonstrated effective acne treatment. | (35) |
| 2 | In vivo/comparative, blinded study | Cream formulation containing cannabis seed extract at 3.0% | - Eleven Asian men (20 - 35 years old); - applied twice daily for three months. | - Erythema decreased by 11.4% on the side treated with the Cannabis cream, while the base cream reduced it by only 4.3%.; - CBD reduced skin sebum by 38%, while the base cream only reduced it by 15%.; - safe, non-allergenic, and well-tolerated with no irritation | (36) |
| 3 | In vitro | CBD solution at 0.5 to 2 μM concentrations | - Applied on normal human epidermal keratinocytes stimulated by C. acnes-derived extracellular vesicles; - analysis of IL-6, IL-8, TNF-α, expression of CB2 receptor and TRPV1 | - CBD suppressed expression of inflammatory cytokines (IL-6, IL-8 and TNF-α); - CB2 receptor expression was upregulated by CBD, whereas CEVs-promoted TRPV1 expression was downregulated by CBD. | (37) |
| 4 | In vivo | Gel containing 1% CBD, 1% Centella asiatica triterpene extract, 1% silymarin and 0.5% of salicylic acid | - 30 subjects (15 - 40 years old) with mild to moderate acne.; - applied 2 - 3 times daily for 56 days. | - Achieved a 70.9% reduction in acne lesions, particularly inflammatory ones. | (38) |
| 5 | In vitro | CBD in DMSO at 10 μM | - CBD in contact with 3D sebocyte gland model and macrophages for 24 hours in a cell incubator | - CBD decreased ROS expression significantly in sebocyte glands in an inflammatory environment;- Sebocyte glands enhance the expression of the CD86 antibody on macrophages by 1.62-fold compared to the control group; - CBD reduced the CD86 signal intensity from 1.62-fold to 1.24-fold. | (39) |
Abbreviation: CBD, cannabidiol.
The results presented demonstrate the potential of CBD as an effective treatment for acne, emphasizing its ability to reduce inflammation, modulate sebocyte activity, and alleviate symptoms such as redness and irritation. In vitro studies have shown that CBD inhibits sebocyte proliferation and lipogenesis while exerting anti-inflammatory effects by suppressing cytokines and regulating immune responses. In vivo studies involving human volunteers further support CBD's efficacy in reducing acne lesions and sebum production when incorporated into topical formulations, often in combination with other ingredients. However, many studies use CBD alongside other active compounds, which complicates isolating its specific effects, highlighting the need for further research to fully understand CBD's role in acne treatment.
3.6. Legislation
Following the legalization of CBD-based medicines, first in the European Union and subsequently in various states of the United States, there has been significant growth and diversification of these products, including those for skincare. However, uncertainties regarding the benefits of skincare products containing CBD or hemp seed oil as active pharmaceutical ingredients persist, primarily due to imprecise formulations. Cannabis emerged as the most popular illicit drug of the twentieth century, and its derivative products are subject to varying international regulations. The use of these substances may be authorized, controlled, or even prohibited in different jurisdictions (14).
However, numerous CBD-containing products claim to offer medicinal benefits — often without supporting evidence. These include capsule supplements for various ailments and cosmetics, such as hemp oils, which are manufactured and distributed without regulatory oversight and may contain unverified ingredients. Currently, there are only a few CBD-based medicines available on the global market; however, none of them are specifically aimed at treating dermatological diseases (14).
In Brazil, the Brazilian Health Regulatory Agency (ANVISA) oversees the procedures for sanitary authorization related to the manufacture, importation, commercialization, prescription, monitoring, and inspection of cannabis-based products intended for medicinal use. These medicines are legalized for medicinal purposes and can only be prescribed to patients diagnosed with epilepsies associated with Dravet and Lennox-Gastaut syndromes, as well as Tuberous Sclerosis Complex. According to RDC 327/2019, products derived from C. sativa must predominantly contain CBD and cannot exceed 0.2% THC. However, patients with diagnoses outside these conditions no longer have access to treatment, which represents a setback for medicinal use and hinders potential scientific advancement and the legalization of CBD for dermatological purposes (40). Cosmetics, tobacco products, and foods based on C. sativa are not classified as medicinal products and are not permitted by law (41).
4. Conclusions
The global outlook on CBD use for skin conditions, including acne, eczema, and dermatitis, is encouraging. The CBD has attracted attention for its valuable pharmacological properties, especially its anti-inflammatory effects in acne treatment. It offers a promising alternative to other treatments like isotretinoin, which can pose various adverse effects on patients. With a favorable safety profile regarding skin physiology and pathology, clinical studies highlight CBD’s effectiveness in reducing sebum production and mitigating inflammation — both of which are linked to the development and worsening of acne.
Nonetheless, further research is needed to expand our knowledge, particularly in understanding the long-term efficacy and safety of CBD in acne treatment, optimizing formulations to enhance skin penetration and bioavailability, and isolating its specific mechanisms of action independent of other active compounds. These directions will support the creation of safe and effective products.