1. Background
Polydeoxyribonucleotide (PDRN) is a DNA extract derived from trout sperm, widely recognized for its regenerative properties. Originally studied over 30 years ago for its potential in treating radiation-induced tissue damage, PDRN has since become an important tool in regenerative medicine and dermatology. Through advancements in purification and extraction techniques, PDRN is now available in a more refined and controlled form, demonstrating significant promise in skincare and wound healing (1, 2).
While therapeutic journey of PDRN began with radiation-related treatments following the Chernobyl disaster in 1986, its uses have expanded far beyond this context. Early studies highlighted its ability to protect against radiation-induced lesions and promote tissue repair (3). More recent research has revealed its potential in various dermatological applications, including anti-aging, wound healing, acne scar treatment, melanogenesis inhibition, and hair regeneration (4, 5).
At its core, PDRN works by stimulating collagen production, promoting angiogenesis (the formation of new blood vessels), and reducing inflammation. The molecular fragments of PDRN, which range from 250 to 1500 kDa, interact with purinergic receptors, driving essential tissue repair processes (6). Clinical studies have shown that when PDRN is administered intradermally, it accelerates wound healing, improves skin elasticity, reduces wrinkles, and even supports hair regrowth (7, 8).
In comparison to conventional treatments, PDRN offers several distinct advantages. For example, while traditional anti-aging therapies like retinoids and Botox primarily focus on smoothing fine lines and wrinkles, PDRN addresses the root causes of aging by stimulating skin regeneration and collagen synthesis. In wound healing, PDRN outperforms other therapies, such as corticosteroids, by enhancing blood flow and speeding up the healing process (9). Additionally, ability of PDRN to reduce inflammation and promote scar remodeling makes it a promising treatment for acne scars, offering a more comprehensive solution compared to methods like laser treatments or chemical peels (10). Its effect on melanogenesis also presents a safer, less invasive alternative to skin lightening treatments, with fewer side effects (11).
The following Table 1 summarizes the effects, mechanisms, and benefits of PDRN in these various cosmetic and therapeutic applications, offering a comprehensive overview of how PDRN supports anti-aging effects, wound healing, scar reduction, inhibition of melanin production, and promotion of hair growth. The table offering a clear framework that will be further discussed in detail in the following sections.
| Effects | Mechanisms | Benefits |
|---|---|---|
| Anti-aging effects | Activation of purinergic receptor A2: PDRN activates the A2a receptor, triggering cell proliferation and supporting growth factors which are essential for tissue regeneration (12). PDRN resists 5′-exonuclease degradation, selectively activating A2a receptors to promote tissue regeneration without broad systemic effects (12). | Activation of purinergic receptor A2: Stimulates collagen and elastin production, improving skin firmness, elasticity, and thickness, and reducing wrinkles (12). |
| Salvage pathway activation: PDRN binds to Adenosine A2a receptors, increasing cAMP levels and activating PKA, which supports cell growth, survival, and tissue repair (12, 13). | Salvage pathway activation: Accelerates wound healing, promotes blood vessel growth, helps fibroblast maturation, and reduces inflammation (12, 13). | |
| Presence of purine and pyrimidine bases: Sodium DNA (PDRN) releases nucleotide bases during DNA degradation, which are essential for cellular repair and DNA metabolism (9). | Presence of purine and pyrimidine bases: Stimulates DNA repair and cellular regeneration, supporting skin cell vitality and promoting anti-aging effects (9). | |
| Cellular uptake and DNA synthesis: Sodium DNA penetrates cell membranes through pinocytosis and endocytosis, aiding DNA and RNA synthesis. This process supports cellular repair and regeneration, especially under stress conditions in aging skin cells like keratinocytes and fibroblasts (9). | Cellular uptake and DNA synthesis: Promotes regeneration of epithelial and granulation tissues, reduces inflammation, and accelerates healing of micro-lesions in the skin (9). | |
| Wound healing effects | Activation of purinergic A2A receptors: PDRN activates the A2A receptor, which plays a central role in cell proliferation, DNA repair, and angiogenesis (12, 14). | Activation of purinergic A2A receptors: Promotes growth of human fibroblasts and osteoblasts, speeding up tissue repair (14). |
| Enhancement of VEGF mRNA expression: PDRN increases the expression of VEGF mRNA. This early induction supports angiogenesis, crucial for the proliferative phase of wound healing (14). | Enhancement of VEGF mRNA expression: Higher VEGF expression leads to increased blood vessel formation, promoting better oxygenation and nutrient supply to wounds (14). | |
| Cell cycle activation (cyclins D1 and E): PDRN activates cell cycle machinery, particularly in damaged or diabetic tissues (14). | Cell cycle activation (cyclins D1 and E): Activation of cyclin-driven cell-cycle progression helps repair wounds, especially in diabetic animals where normal cell-cycle function is impaired (14). | |
| Augmentation of microvessel density (CD31 immunostaining): PDRN significantly enhances microvessel density in wound tissues, as indicated by increased CD31 immunostaining. This effect supports the formation of new blood vessels (angiogenesis), which is critical for delivering oxygen and nutrients necessary for tissue repair (14). | Augmentation of microvessel density (CD31 immunostaining): Increases dermal and epidermal regeneration, promoting faster re-epithelialization and reducing scarring (14). | |
| Enhancement of tensile strength of diabetic wounds: PDRN improves the tensile strength of diabetic wounds during the healing process. The regenerated tissue is stronger and more resilient, which is important for preventing wound dehiscence and promoting overall wound closure (14). | Enhancement of tensile strength of diabetic wounds: Stronger wound healing, PDRN-treated diabetic wounds exhibit greater tensile strength, improving structural integrity and reducing complications (14). | |
| Anti-acne scars effects | Suppression of inflammatory cytokines (IL-6, TNF-α, HMGB-1): PDRN inhibits the release of pro-inflammatory cytokines such as IL-6 and TNF-α, which are key mediators of the inflammatory response. HMGB-1, a DAMP, is also suppressed by PDRN (15-17). | Suppression of inflammatory cytokines (IL-6, TNF-α, HMGB-1): Reduces inflammation, promoting faster wound healing and reduced scarring (15-17). |
| Inhibition of mast cell degranulation: PDRN modulates immune responses by inhibiting mast cell degranulation. This prevents the release of histamine and other inflammatory mediators, which normally contribute to prolonged inflammation and excessive scarring (16, 17). | Inhibition of mast cell degranulation: Decreases the release of pro-inflammatory mediators, limiting tissue damage and reducing scar formation (16, 17). | |
| Collagen synthesis modulation: PDRN promotes the synthesis of collagen, an essential component of the extracellular matrix. By facilitating collagen production in a controlled manner, PDRN accelerates wound closure and reduces the appearance of scars (18). | Collagen synthesis modulation: Enhances collagen production, improving wound healing and minimizing the appearance of scars (18). | |
| Promotion of cell proliferation and angiogenesis: PDRN activates the A2A adenosine receptor, which triggers pathways leading to cell proliferation (e.g., fibroblasts and ECs) and angiogenesis (formation of new blood vessels) (19, 20). | Promotion of cell proliferation and angiogenesis: Stimulates cellular regeneration and new blood vessel formation, enhancing tissue repair and accelerating healing by improving issue oxygenation and nutrient delivery (19, 20). | |
| Anti-melanogenesis effects | Activation of adenosine A2A receptors: Stimulates the phosphorylation of ERK and AKT. These pathways play a role in modulating melanogenesis and skin pigmentation (21). | Activation of adenosine A2A receptors: Reduces pigmentation by regulating melanogenesis signaling pathways (21). |
| Downregulation of MITF: The MITF is a transcription factor that activates the genes responsible for melanin synthesis, including tyrosinase, TRP-1, and TRP-2. PDRN reduces MITF levels, thereby suppressing melanin production (22). | Downregulation of MITF: PDRN inhibits melanogenesis by reducing melanocyte activation (36). | |
| Suppression of tyrosinase activity: PDRN decreases intracellular tyrosinase activity, a key enzyme in the melanogenic pathway. By inhibiting tyrosinase, PDRN reduces melanin synthesis, which leads to lighter skin pigmentation (21). | Suppression of tyrosinase activity: PDRN reduces melanin production, preventing hyperpigmentation (21). | |
| Anti-hair loss effects | Activation of follicular regeneration pathways PDRN stimulates growth factors involved in follicular regeneration, which leads to the thickening of existing hair shafts and a general improvement in hair follicle health (23). | Both PDRN alone and combined PDRN/PRP treatments increased hair thickness and hair count compared to PBS controls, although no significant difference was noted between the two PDRN and PDRN/PRP treatments in hair count (23). |
| Synergistic effect with PRP: The combination of PDRN with PRP showed superior results compared to PDRN alone, especially in increasing hair thickness, although both treatments improved hair count compared to controls (23). |
Summary of the Effects, Mechanisms, and Benefits of Polydeoxyribonucleotide in Cosmetic Applications
2. Methods
A narrative review of the literature was conducted to explore the therapeutic effects of PDRN in dermatology and cosmetology. The search was performed using PubMed, Scopus, and Google Scholar, covering studies published within the last two decades. Crucial terms similar as "Polydeoxyribonucleotide", "Wound Healing", "Aging", "Hair Growth", "Collagen", "Acne Scar", "Purinergic Receptors", "Platelet-Rich Plasma" and "Regenerative Medicine" were used to identify applicable articles.
Addition criteria for the review were clinical and preclinical studies that investigated the molecular and clinical effects of PDRN in the fields of wound healing, anti-aging, hair regeneration, acne scar treatment, and melanogenesis. Studies were selected based on their relevance to the topic and the quality of the evidence presented. Papers published in English were included, while studies unrelated to PDRN or those with insufficient data were excluded.
The review did not follow a formal protocol or predefined quantitative methodology, allowing for a broader, qualitative synthesis of the available literature.
3. Anti-aging Effects
3.1. Aging Process
Skin aging is a gradual process characterized by the progressive degeneration of skin tissue and visible changes to the skin’s surface. As wrinkles form and deepen, the skin loses its firmness, elasticity, and thickness, particularly in the epidermal layer. These alterations signal the transition from youth to old age. While visible signs of aging often appear after the age of 30, significant cellular and structural changes can begin earlier, influenced by factors such as hormones, genetics, metabolism, environment, and overall biology (9).
The most prominent sign of aging is the skin’s diminished ability to retain moisture, coupled with a reduction in dermal elasticity. This becomes particularly evident as facial muscles contract, causing wrinkles to deepen (9). Over time, biological and environmental damage accumulate, and natural repair mechanisms become less effective. The most notable changes occur in collagen and elastin fibers, key components of connective tissue. A decrease in collagen synthesis by fibroblasts, driven by factors like solar radiation and hormonal changes (e.g., estrogen decline during menopause in women), significantly contributes to the visible signs of aging (9).
3.2. Mechanism of Action of Polydeoxyribonucleotide
The anti-aging effects of Sodium DNA (or PDRN) are likely due to the presence of purine and pyrimidine bases released during DNA degradation, which are essential for cellular vitality and repair (8). As PDRN degrades, these nucleotide bases can be utilized by cells for DNA repair, nucleic acid synthesis, and other metabolic processes. The DNA itself can penetrate cell membranes via pinocytosis and endocytosis, facilitated by sodium ions that interact with PDRNs. Once inside the cell, the released nucleotide bases can support the synthesis of new DNA or RNA, which is crucial for cellular functions such as repair and regeneration, particularly under metabolic or extreme stress conditions, such as those encountered in aging skin cells like keratinocytes and fibroblasts (9).
Through these mechanisms, PDRN stimulates the repair of damaged cells, promotes the regeneration of epithelial and granulation tissues, reduces inflammation, and accelerates the healing of micro-lesions in the skin (9). In vitro studies on human epidermal keratinocytes and fibroblasts, using the 3T3 Neutral Red Uptake method, demonstrated that nucleotide fragments of PDRN, contained in a proprietary formulation known as DNA-Na, exhibit protective and regenerative effects. These effects translate into significant improvements in key skin parameters, including moisturization, elasticity, thickness, and wrinkle reduction (9).
Although it’s extensively known that nucleotides and their derivatives can increase cell proliferation in vitro, the exact mechanisms behind this remain unclear. One of the better-ideas is that nucleotides activate the purinergic A2 receptor, which plays a crucial part in cellular responses. When this receptor is activated, it can trigger a proliferative response in cells, potentially working together with growth factors to promote tissue regeneration. Research has demonstrated that the A2 receptor is involved in processes like inflammation, wound healing, and cell growth, which all are important for skin regeneration and repair (10, 11).
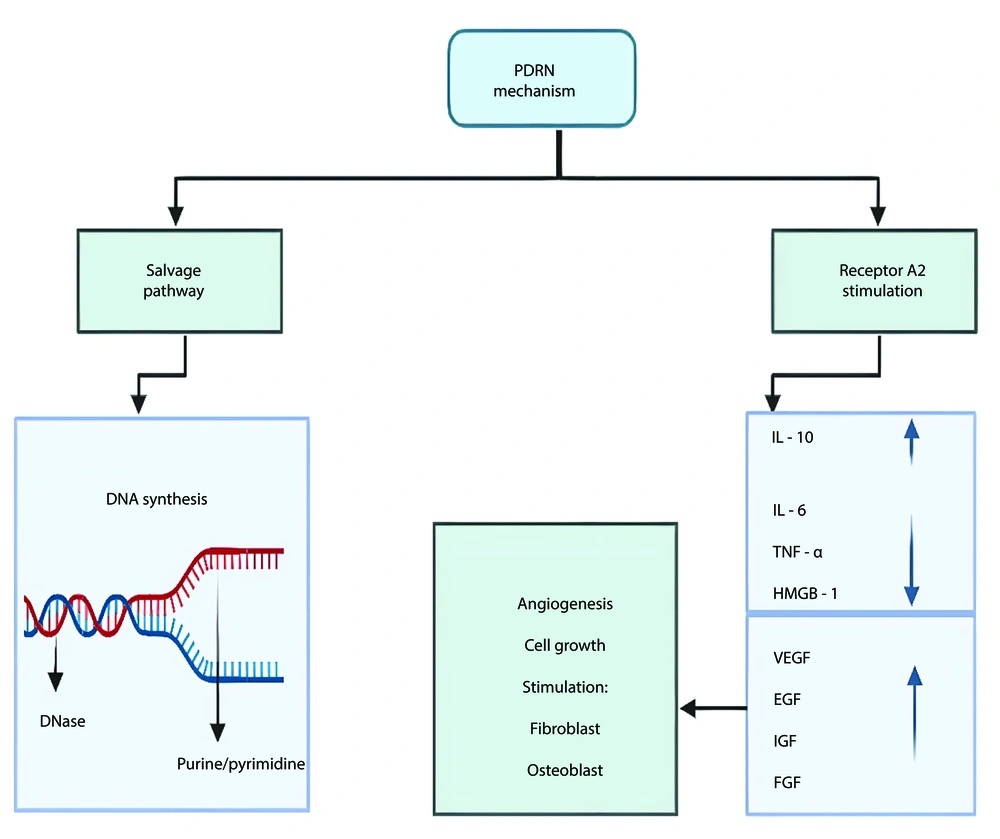
Polydeoxyribonucleotide promotes wound healing by interacting with Adenosine A2a receptors and triggering a process known as the salvage pathway. When PDRN binds to these receptors, it increases levels of cAMP, which then activates protein kinase A (PKA), influencing cell functions like growth, survival, and repair (12, 13). This action supports healing by blood vessel growth, helping fibroblasts mature, and reducing inflammation. The role of A2a receptors was confirmed by the fact that blocking them with a specific antagonist, DMPX, stops these healing effects. Polydeoxyribonucleotide has the unique ability to resist 5′-exonuclease degradation, allowing it to selectively activate A2a receptors without causing the widespread effects seen with other adenosine compounds. Polydeoxyribonucleotide also plays a role in tissue regeneration by supporting DNA repair and cell growth through the salvage pathway, making it a powerful tool for healing and recovery (12, 24) (Figure 1).
4. Wound Healing Effects
4.1. Wound Healing Process
Wound healing is a complex, dynamic process that involves three interrelated stages: The inflammatory phase, the proliferative phase, and the remodeling phase. The initial stage focuses on achieving hemostasis, followed by the recruitment of leukocytes and monocytes to the wound site during the inflammatory phase, which can be further divided into vascular and cellular responses (10). During this phase, white blood cells produce growth factors that prepare the wound for the subsequent proliferative phase, where fibroblasts and endothelial cells (ECs) are recruited. In this stage, new collagen and ground substances are synthesized to restore wound integrity, while angiogenesis (25, 26) ensures a sufficient supply of nutrients to support the newly forming tissue. Over several months, the scar undergoes remodeling and maturation, completing the wound healing process.
4.2. Mechanism of Action of Polydeoxyribonucleotide
Polydeoxyribonucleotide has shown significant promise in promoting wound healing across various injury types, with its effectiveness varying depending on the nature of the wound and underlying health conditions.
Efficacy in different wound types:
(1) Diabetic wounds: Polydeoxyribonucleotide accelerates wound healing by increasing the expression of VEGF (vascular endothelial growth factor), promoting blood vessel formation, and enhancing tissue oxygenation. In diabetic mice, PDRN activates the cell cycle, boosting cyclins D1 and E, crucial for cell-cycle progression (14).
Key study findings:
(1) VEGF expression: Polydeoxyribonucleotide increased VEGF expression in diabetic mice, improving angiogenesis (P < 0.05) (14); (2) microvessel density: CD31 staining revealed increased microvessel density in both normoglycemic and diabetic mice treated with PDRN (14); (3) tensile strength: Diabetic wounds treated with PDRN had significantly higher tensile strength (P < 0.01), suggesting better wound integrity (14).
Polydeoxyribonucleotide accelerates healing by reducing wound diameter and improving angiogenesis (14).
(2) Corneal injuries: A study by Edirisinghe et al. developed a corneal epithelial injury model in zebrafish (Danio rerio) to evaluate the effectiveness of PDRN in promoting corneal healing. Chemical injury was induced using a 3% acetic acid solution, and PDRN treatment was administered by immersing the zebrafish in PDRN-containing water at various time points post-injury. Results showed that PDRN significantly accelerated corneal re-epithelialization, reducing the wound area at 48- and 72-hours post-injury. Histological analysis revealed improved epithelial cell organization and increased goblet cell density. Additionally, PDRN treatment upregulated key genes involved in healing, such as adenosine A2b receptor (ADORA2B), paired box 6A (PAX6A), paired box 6B (PAX6B), and mucin 2.1 (MUC2.1), and modulated inflammatory markers like tumor necrosis factor-alpha (TNF-α) and heat-shock proteins (HSP70, HSP90AB1). Immunoblotting confirmed the modulation of proteins such as matrix metalloproteinase-9 (MMP-9), HSP70, and TNF-α. This study highlights the potential of PDRN in promoting corneal epithelial regeneration and wound healing. (27, 28).
(3) Full-thickness skin defects: A study by Lee et al., compared the wound healing effects of PDRN derived from Oncorhynchus mykiss (trout) and Oncorhynchus keta (salmon) in different formulations. Full-thickness skin defects were created in mice, and the healing effects of O. mykiss-derived PDRN injection, O. keta-derived PDRN injection, O. keta-derived PDRN cream, and normal saline (control) were assessed over 10 days. The results showed that both O. keta-derived PDRN injection and O. mykiss-derived PDRN injection exhibited the greatest wound healing effects, with similar outcomes. The PDRN solution (group I) also showed superior efficacy compared to other treatments, with the greatest reduction in wound size, enhanced re-epithelialization, and granulation tissue formation. Notably, O. keta-derived PDRN injection reduced the time required for wound healing, suggesting it may serve as an effective alternative to the current clinical standard. Based on these findings, PDRN-soaked dressings, especially O. keta-derived PDRN, should be considered a preferred option for treating full-thickness skin defects (29, 30).
Factors influencing efficacy:
(1) Molecular weight: The study by Hwang et al., investigated the effectiveness of different molecular weights of PDRN in promoting skin wound healing in mice. Polydeoxyribonucleotide was categorized into low (< 50 kDa), middle (50 - 1,500 kDa), and high (> 1,500 kDa) molecular weights. The results showed that classic PDRN (50 - 1,500 kDa) was most effective in improving wound healing quality. Histological analysis revealed less lipid accumulation and more collagen formation in this group. Additionally, classic PDRN enhanced cell migration through c-Jun N-terminal kinase signaling in human fibroblasts. These findings suggest that medium molecular weight PDRN (50 - 1,500 kDa) is optimal for wound healing and tissue regeneration (31).
(2) Wound environment: Factors like pH, infection, and oxygenation can affect performance of PDRN (32).
(3) Health conditions: Underlying diseases such as diabetes, cardiovascular problems, and obesity may impair natural healing, thereby impacting the efficacy of PDRN. Additionally, certain medications can also affect the healing process and influence the effectiveness of PDRN treatment (19).
5. Anti-acne Scars Effects
5.1. Formation of Acne Scars
Acne scars develop as a result of a complex factors, including increased sebum production, changes in sebum lipid composition, androgen activity, and the proliferation of Cutibacterium acnes (C. acnes) within the hair follicles (33). The C. acnes can trigger an inflammatory response by activating keratinocytes (skin cells) and sebocytes (sebaceous gland cells) through interactions with toll-like receptors (TLRs), which are proteins on the surface of cells that play a key role in immune response, as well as CD14, a co-receptor involved in recognizing pathogens, and CD1, which is involved in lipid antigen presentation (20). Additionally, macrophages expressing TLR2 surround the pilosebaceous follicles (hair follicle and sebaceous gland) in acne lesions. When TLR2 is activated, it triggers the nuclear transcription factor NF-κB, which leads to the production of pro-inflammatory cytokines and chemokines. These molecules play key roles in inflammation. Furthermore, C. acnes can stimulate the release of cytokines such as interleukin-8 (IL-8) and interleukin-12 (IL-12) from TLR2-positive monocytes, further increasing the inflammatory response (34). This inflammation, combined with skin damage during the healing process, contributes to the formation of acne scars (35).
5.2. Mechanism of Action of Polydeoxyribonucleotide
Polydeoxyribonucleotide produces its beneficial effects through multiple mechanisms. Suppression of inflammatory cytokines and inhibition of mast cell degranulation is primarily responsible for its anti-inflammatory effects (15, 16). Previous studies have shown that PDRN therapy reduces the levels of pro-inflammatory mediators, such as interleukin 6 (IL-6), TNF-α, and HMGB-1 (15) (Figure 1). Although PDRN has been observed to decrease scarring indirectly by modulating inflammatory responses, the direct relationship between PDRN and scarring has yet to be fully explored in dedicated studies (17).
Jeong et al. (36) assessed the anti-inflammatory effects of PDRN on scar formation. In these studies, 30 Sprague-Dawley rats were subject to dorsal skin excision and the subsequent repair process evaluated. Test animals were divided into three groups: A PDRN-3 group (n = 8), which received PDRN intraperitoneally at a dose of 8 mg/kg for three days; a PDRN-7 group (n = 8), which received the same dose for seven days; and a PDRN-3+HMGB-1 group (n = 6), which received HMGB-1 via intradermal injection along with PDRN for three days. The efficacy of PDRN in reducing scarring was evaluated using histological analysis and immunohistochemistry, focusing on inflammatory cell counts and internal scar area. PDRN treatment significantly reduced scar area, inflammatory cell infiltration, and the presence of CD45-positive cells. Notably, PDRN also decreased the expression of HMGB-1, a critical inflammatory mediator that was elevated in the sham group. Conversely, when HMGB-1 was administered alongside PDRN, the benefits of PDRN on collagen synthesis were reversed, and inflammatory profiles resembled those of the sham group. These findings indicate that PDRN exerts its anti-inflammatory and collagen-modulating effects primarily through HMGB-1 suppression, leading to accelerated wound healing and diminished scar formation. Thus, PDRN represents a promising therapeutic approach for managing scar tissue development following skin injuries (32).
6. Anti-melanogenesis Effects
6.1. Process of Melanogenesis
In the skin, melanocytes, fibroblasts, and keratinocytes regulate pigmentation through juxtracrine and paracrine signaling. Keratinocytes play a pivotal role in this process by secreting melanogenic and proliferative factors that bind to specific receptors on melanocytes. Melanogenesis involves multiple biochemical pathways and the coordinated effects of several enzymes. Tyrosinase is a rate limiting enzyme in these pathways. This glycoprotein, which contains copper, is essential for melanin production. Other important enzymes in the melanogenic pathway include TRP- 1 and TRP- 2. Also, the transcription factor, microphthalmia-associated transcription factor (MITF), is essential to regulating the expression of melanocyte-specific enzymes (18, 37, 38).
6.2. Mechanism of Action of Polydeoxyribonucleotide
Polydeoxyribonucleotide has been shown to inhibit melanogenesis through several important mechanisms. Studies by Noh et al., demonstrated that PDRN can significantly reduce melanin synthesis in the immortal Murine melanocyte cell line, Mel-Ab, as well as in human melanocyte-keratinocyte cocultures. A notable decrease in melanin content was observed when these cells were treated with PDRN (10 - 200 μg/mL) or Placentex® (10 - 100 μg/mL) over a four-day period, indicating a dose-dependent inhibition of melanogenesis (21).
Polydeoxyribonucleotide exerts at least some of its effects on melanogenesis by suppressing intracellular tyrosinase activity. Findings suggest that Mel-Ab cells treated with varying concentrations of PDRN or Placentex® for four days showed a significant reduction in tyrosinase activity (22). Polydeoxyribonucleotide also appears to downregulate the expression of MITF and other proteins that are also involved in melanogenesis. The MITF plays a pivotal role in activating the transcription of tyrosinase and its related proteins, including TRP-1 and TRP-2. Treatment with PDRN or Placentex® for 24 to 72 hours has been shown to decrease levels of MITF, tyrosinase, and TRP-1 in Mel-Ab cells (22). This reduction contributes to the overall inhibition of melanin production.
Another key aspect of PDRN’s mechanism involves the activation of adenosine A2A receptors. This activation stimulates the phosphorylation of ERK and AKT, which may be important in mediating PDRN’s anti-melanogenic effects (21).
7. Anti-hair Loss Effects
Hair loss is a common issue that affects approximately 50% of both men and women during their lifetime. It can occur in various parts of the body, but it predominantly affects the scalp, often leading individuals to seek treatment due to its cosmetic impact (39). Hair loss can be broadly categorized into three types: Hair shaft abnormalities, permanent alopecia, and nonpermanent alopecia. Among these, nonpermanent alopecia is the most prevalent, and it includes conditions such as androgenetic alopecia, telogen effluvium, alopecia areata, and traction alopecia (40).
7.1. Process of Female Pattern Hair Loss
Female pattern hair loss (FPHL) is a subtype of androgenetic alopecia that results in progressive thinning of hair without scarring. It occurs due to a reduction in the ratio of terminal hairs (thick, fully-grown hairs) to shorter, finer vellus hairs, a process known as follicular miniaturization (41). The FPHL typically follows a specific pattern of hair loss, with the frontal and vertex areas of the scalp being most commonly affected, though the parietal and occipital regions may also show signs of thinning (42).
7.2. Mechanism of Action of Polydeoxyribonucleotide
A study conducted by Lee et al. assessed the effectiveness of intra-perifollicular injections of autologous platelet-rich plasma (PRP) and PDRN for treating FPHL. In this study, one group of 20 FPHL patients received a single PRP treatment on their scalps, followed by 12 weekly sessions of PDRN injections. A second group of 20 FPHL patients received only the 12 weekly treatments of PDRN. A control group for each study received phosphate-buffered saline (PBS) in place of PRP and PDRN. Tissue samples from each group of rabbits were analyzed using real-time polymerase chain reaction (PCR) and Western blotting techniques. Significant improvements were observed in hair count and hair thickness in patients who received the combined PRP and PDRN treatment as well as the groups containing only PDRN injections compared to the PBS control group. However, groups receiving the combined therapy demonstrated a more significant increase in hair thickness than those treated solely with PDRN (P = 0.031). No notable difference was found in hair count between combined therapy and those who received solely PDRN (P > 0.05) (23).
The frequency of PDRN injections in this study was set at 12 weekly sessions, and this duration was sufficient to yield measurable results in hair regrowth. As noted in other studies, patients typically observe initial improvements within 4 - 6 weeks, with optimal results occurring after 2 - 3 months of consistent treatment. In clinical practice, treatments may vary in frequency based on the severity of the condition and the individual’s response to therapy.
8. Discussion
Polydeoxyribonucleotide has shown significant potential as a therapeutic agent due to its anti-inflammatory, angiogenic, and tissue-regenerating properties. These qualities make it an attractive alternative or complement to existing therapies. However, despite promising initial results, several limitations need to be addressed before PDRN can be widely used in clinical settings.
One major limitation is the variability in outcomes seen across studies. While many clinical trials report positive results, the effectiveness of PDRN seems to differ greatly between individuals. Factors such as the severity of the condition, individual response, and patient adherence to the treatment regimen can all influence the therapeutic benefit. Additionally, although PDRN has been shown to improve wound healing and scar reduction, the degree of these effects is still not entirely clear, with some studies indicating only modest improvements. This variability underlines the need for more controlled and long-term studies to establish consistent, reliable results.
Another gap in the current research is the lack of clarity on the frequency and duration of PDRN treatments needed for optimal outcomes, particularly in hair regrowth and wound healing. The optimal dosing schedule has not been well studied, and more research is needed to determine the most effective treatment regimens to achieve lasting results. Without a clear understanding of these factors, it may be difficult for clinicians to use PDRN effectively in practice.
The mechanism behind PDRN’s action is also still being explored. Research suggests that its therapeutic effects are primarily due to its purine and pyrimidine content, which activate purinergic A2A receptors, triggering pathways related to angiogenesis and fibroblast activation. However, there are still unanswered questions about the role of PDRN fragment size and nucleotide sequence in determining the specificity and potency of its effects. Further studies are needed to better understand how these factors influence therapeutic outcomes.
In terms of safety, PDRN has shown excellent tolerability. Studies in animals and humans have shown no significant toxicity in critical organs such as the brain, heart, liver, or lungs following systemic administration. Clinical trials evaluating PDRN for conditions like diabetic foot ulcers and skin rejuvenation have also reported good safety profiles, with no severe adverse effects or immunological reactions. These findings support the use of PDRN in various medical applications.
Despite its promising therapeutic potential, the clinical use of PDRN is limited by its high cost and complex production process. Extracting and purifying PDRN from sperm requires specialized techniques, making it more expensive than traditional treatments. Research into cost-effective production methods and exploring alternative delivery systems could help make PDRN more accessible to a wider range of patients.
Looking ahead, future research should focus on conducting large-scale, longitudinal clinical trials to validate PDRN’s efficacy and safety in diverse patient populations. In-depth mechanistic studies are also needed to clarify how PDRN fragments interact with cellular receptors and signaling pathways, which could help optimize treatment protocols. Additionally, investigating the potential of combining PDRN with therapies like PRP, stem cells, or other growth factors may enhance therapeutic outcomes, as early studies suggest such combinations may have synergistic effects.
8.1. Conclusions
In conclusion, PDRN is a promising therapeutic agent with the potential to revolutionize treatments in cosmetology and regenerative medicine. Its multifaceted biological effects make it a compelling candidate for a variety of indications, from wound healing, anti-aging to scar reduction and hair restoration. However, significant gaps remain in understanding its molecular mechanisms, and much work is needed to refine its clinical applications. With continued research and development, PDRN holds the potential to transform not only the field of dermatology but also broader areas of regenerative medicine.