1. Introduction
Bullous pemphigoid (BP) and lupus erythematosus (LE) are both autoimmune diseases characterized by circulating antibodies targeting different antigens. Although they may be associated with each other, they rarely present with coexisting or overlapping features, with only a few cases reported to date in the literature (1). Bullous pemphigoid is a subepidermal vesiculobullous disease that typically affects older individuals. It presents with subepidermal blisters with a predominantly eosinophilic infiltrate and linear deposits of C3 and IgG at the basement membrane zone on direct immunofluorescence. Vesicles and blisters in lupus erythematosus are either secondary to vacuolar epidermal changes or antibodies against type VII collagen with neutrophilic aggregates. We herein report a case of a woman with the coexistence of lupus erythematosus and bullous pemphigoid and elaborate on the diagnostic and management challenges.
2. Case Presentation
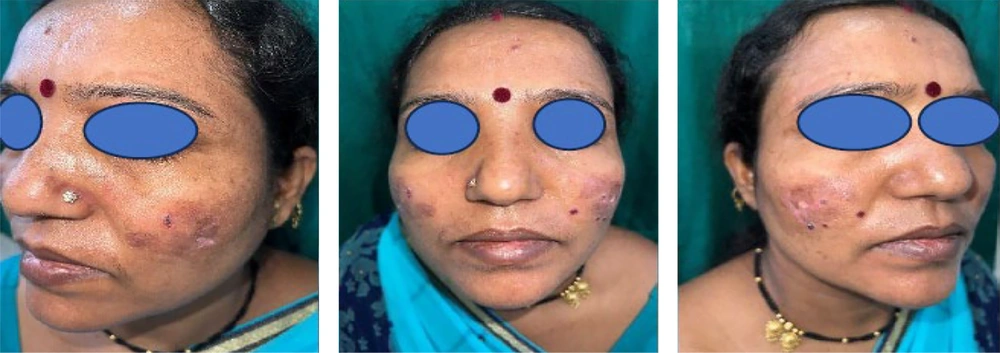
A 41-year-old woman visited the Dermatology Out-patient department with blisters over the malar area and trunk, associated with severe itching and burning for 1 month. She reported a history of difficulty in eating, particularly intolerance to spicy food, with recurrent oral mucosal erosions. A history of photosensitivity was present. She denied any history of fever, joint pain, Raynaud’s phenomenon, erosions at trauma-prone sites, burning micturition, ocular redness, recent drug intake or vaccination, or similar complaints in family members. On examination, her vital parameters were stable, with no pallor, icterus, cyanosis, or edema. Systemic examination was unremarkable. On dermatological examination, there were a few hemorrhagic vesicles arising over an erythematous base and crusting predominantly over the malar area, forehead, and upper chest. Erythematous to violaceous plaques with fine adherent scales, telangiectasias, and atrophy over the bilateral malar area were noted (Figure 1). Erythema and telangiectasias were seen on the nasolabial folds. The Asboe-Hansen sign was positive, the Lutz sign showed a regular rounded border, and the Nikolsky sign was negative. Erosions were noted over the buccal mucosa, hard and soft palate. Post-inflammatory hyperpigmentation with central hypopigmentation and atrophic scars were present over the abdomen. Clinical differentials considered were bullous lupus erythematosus, bullous pemphigoid with mucosal involvement, and bullous lichen planus.
Complete hemogram (CBC), renal function test (RFT), liver function tests (LFT), erythrocyte sedimentation rate (ESR), peripheral blood smear (PBS), and urine routine microscopy (URM) were within normal limits, except for a raised absolute eosinophil count (630 cells/cumm). C-reactive protein and rheumatoid factor were negative. The 24-hour urine protein was 15 mg/24 hrs (normal < 300 mg/24 hrs). Serological viral markers (Human Immunodeficiency Virus, Anti-Hepatitis C Virus, Hepatitis B Antigen) were non-reactive. X-ray (chest), ultrasound, and electrocardiogram (ECG) were unremarkable. A skin biopsy from the vesicle showed a subepidermal blister with dense polymorphs and eosinophils. The antinuclear antibody test (by indirect immunofluorescence method) was positive in significant titers (1:320) with a speckled and cytoplasmic speckled pattern. However, the specific antibody profile by the ELISA method was within normal limits. A direct immunofluorescence (DIF) study of perilesional skin revealed IgG focal positivity at the dermo-epidermal junction, but IgM, IgA, and C3 were negative. A final diagnosis of Lupus erythematosus – Bullous Pemphigoid overlap was made. After a short course of oral prednisolone, she was started on dapsone 100 mg once daily with CBC and LFT monitoring, considering the effectiveness of this drug in both bullous eruption of lupus erythematosus and bullous pemphigoid. Oral hydroxychloroquine 200 mg/day (after ophthalmic and cardiac evaluation) was given along with topical sunscreen. She responded to this treatment with marked improvement in symptoms and lesions, in the form of reduced photosensitivity and complete resolution of vesicles with no appearance of new lesions. After 1 month, her hemoglobin declined; hence, dapsone was withheld, and she was maintained on oral hydroxychloroquine 200 mg twice a day with adequate sun protection. Currently, she is under regular follow-up for one year with occasional crops of oral erosions, which respond satisfactorily to triamcinolone buccal paste. There have been no further episodes of malar rash and vesiculo-bullous lesions.
3. Discussion
Subepidermal immunobullous disease (SIBD) comprises BP, cicatricial pemphigoid (CP), epidermolysis bullosa acquisita (EBA), pemphigoid gestationis (PG), linear IgA dermatosis (LAD), dermatitis herpetiformis (DH), and bullous systemic lupus erythematosus (BSLE). LE-associated vesiculobullous diseases have variable presentations and are classified as (1) LE-specific [acute cutaneous LE (TEN-like ACLE), SCLE (TEN-like SCLE and vesiculobullous annular SCLE), and chronic CLE (bullous discoid LE)], (2) aspecific (Bullous SLE), (3) LE-related autoimmune bullous diseases (vesiculobullous skin disorders reported to occur concomitantly in LE patients, e.g., PV, PF, BP, DH, and PCT), and 4) LE in association with non-autoimmune conditions (EM, SJS/TEN, and PCT)(2,3). Vesicles and blisters in LE may occur due to (1) an interface vacuolar dermatitis; (2) antibodies like anti-collagen VII (related to an autoimmune blistering disease seen in bullous systemic lupus erythematosus); (3) co-existence of LE with other autoimmune blistering diseases (e.g., bullous pemphigoid). Up to 30% of SLE patients have an additional underlying autoimmune disease (2). Both pemphigus and pemphigoid are documented to occur with numerous other autoimmune conditions, e.g., connective-tissue diseases, particularly SLE, myasthenia gravis, thymoma, and chronic thyroiditis (1). The BSLE is an infrequently encountered variant of systemic lupus erythematosus and is often accompanied by lupus nephritis (3). The vesicles and bullae develop rapidly and are more widespread as symmetrically distributed erythematous plaques and papules, seen over the face, neck, and upper trunk. Oro-pharyngeal, ocular, laryngeal, and genital mucosae may be involved. Lesional progression and healing usually occur with milia, hypo- or hyperpigmentation without scarring. Biopsy of BSLE is characterized by a subepidermal bulla with a neutrophil-predominant inflammatory infiltrate and may resemble dermatitis herpetiformis or inflammatory epidermolysis bullosa acquisita. The DIF shows linear IgG deposits at the BMZ with sparse granular IgM, IgA, and C3 (2). Indirect immunofluorescence (IIF) may detect autoantibodies against type VII collagen (2). Dapsone is effective in the treatment of bullous LE. The BP is a subepidermal blistering disorder with a predilection for elderly individuals. It presents as severely pruritic tense bullae usually on a urticarial base involving flexures. Typical histopathology shows a subepidermal blister with an inflammatory infiltrate composed of eosinophils and polymorphs. DIF studies on normal-appearing perilesional skin reveal IgG antibodies in the majority of cases and C3 in 100% of cases at the basement membrane zone (4). IIF studies document IgG (subclass IgG4) circulating autoantibodies against BP180 and BP230 in the patient’s serum. The salt split technique of immunofluorescence has recently been described in the evaluation of subepidermal blistering disorders, which shows a pattern of linear roof and floor fluorescence to differentiate bullous pemphigoid from EBA and bullous LE (collagen VII). BP is successfully treated with immunosuppressants like dapsone, cyclophosphamide, azathioprine, cyclosporine A, methotrexate, combination nicotinamide along with tetracycline/minocycline, and mycophenolate mofetil (MMF).
LE–BP overlap is characterized by a combination of clinical, histological, and/or immunological features of both diseases in the same patient. In our case, severe itching, vesicles over an erythematous base with positive Asboe-Hansen sign and Lutz sign were in favor of BP. Oral erosions may be encountered in both BP and LE but are more predominantly seen in LE. Photosensitivity, malar area involvement, atrophic scars, and telangiectasias over the involved area were classical of LE. She had peripheral eosinophilia and characteristic histopathological features of bullous pemphigoid with a few features suggestive of LE (neutrophilic infiltrate with periadnexal lymphocytic infiltrate) while demonstrating positive serological markers (significant ANA titer of 1:320) of lupus erythematosus. Therefore, to differentiate between these two specific disorders, we performed a DIF study which showed IgG focal positivity at the dermo-epidermal junction, which can be seen in both BP and LE. As LE-BP overlap syndrome has not been detailed in the literature and clear diagnostic criteria do not currently exist, after analyzing all investigations, we arrived at the diagnosis of coexisting or overlapping features of cutaneous LE and BP. A probable explanation of the coexistence of LE and BP is the ‘epitope spreading’ phenomenon, which states that targets of immune responses in autoimmunity do not remain fixed but can be expanded to include other antigens on the same protein or other proteins in the same tissue (5). The “epitope spreading” phenomenon also pertains to situations in which a previously hidden (“sequestered”) antigen is exposed subsequent to tissue damage resulting from a primary inflammatory process, further culminating in a secondary autoimmune response against the newly released antigen. In our case, the primary inflammatory process is due to interface dermatitis (seen in LE). This inflammation probably caused damage to the dermo-epidermal junction (DEJ) with consequent exposure of previously sequestered dermal antigens. This led to a secondary autoimmune response and development of antibodies against the dermo-epidermal junction, manifesting as BP. Maggio et al. (1) have reported an 11-year-old girl who presented with tense bullous skin lesions with arthralgia and mild proteinuria. She was diagnosed with SLE associated with BP on the basis of clinical and histopathologic features and persistent high titers of anti-dsDNA and ANA. However, in contrast, predominant systemic involvement was not seen in our case. Although ANA was positive in significant titers (speckled pattern), the ANA-blot was negative. Their patient showed drastic improvement with dapsone and a short course of systemic steroids similar to the present case. Cutaneous lupus lesions show a good response to hydroxychloroquine by immunomodulatory effects and acting as a systemic sunscreen. Although the use of dapsone is rarely reported (bullous eruption of LE shows dramatic response) (6), it can be an effective modality in lupus erythematosus owing to its anti-inflammatory properties related to the inhibitory effect on neutrophil-mediated tissue damage by converting myeloperoxidase into an inactive compound, thereby inhibiting toxic oxygen intermediates. Dapsone, acting by a similar mechanism as in LE, is an established modality for lesions of bullous pemphigoid (particularly cell-rich variant as confirmed by histopathology in our patient). Thus, the combination of hydroxychloroquine and dapsone may be expected to be very useful in the scenario of LE-BP overlap.
3.1. Conclusions
Autoimmune diseases encompass a broad spectrum of disorders, and severe forms can present with life-threatening consequences. It is recommended that healthcare providers are able to comprehend and recognize atypical variants and combinations of autoimmune disorders based on clinical, histopathological, and/or immunopathological information to establish a definitive diagnosis. Coexistence of LE and BP is exceedingly rare and should be dealt with higher suspicion. Diagnosis remains difficult attributable to similarities in clinical and immunohistopathological features.