1. Introduction
Viral exanthems pertain to eruptions over the skin occurring due to viral infections. Exanthema usually follows a typical pattern for specific infections and may be associated with systemic features. Hand-foot-mouth disease (HFMD) is a common childhood viral exanthema. It is very infrequently encountered in adults; however, the exact number of cases has not been documented so far. Most often, the diagnosis is clinical. Occasionally, the presentations can be atypical, especially in adult individuals with pre-existing comorbidities, leading to diagnostic difficulty. In such cases, vigilant observation, appropriate laboratory evaluation, and stringent follow-up are mandatory. This case report describes an extremely rare presentation of this common childhood viral exanthem in an immunocompromised adult and attempts to highlight the challenges involved in the diagnosis and management of such florid presentations.
2. Case Presentation
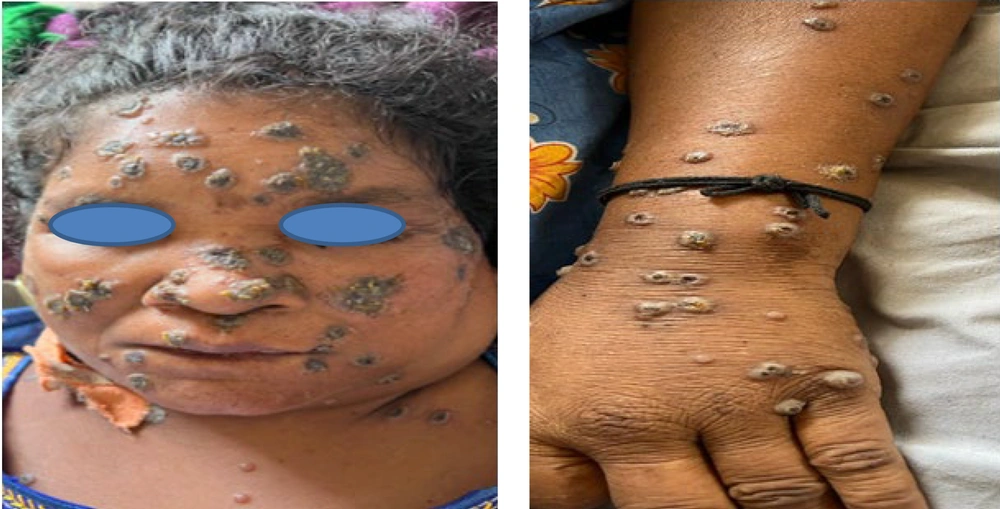
A 50-year-old woman undergoing treatment at the general medicine department in a tertiary health care center was referred for dermatology consultation due to multiple asymptomatic fluid-filled lesions over the body for 1 week, associated with fever. The lesions were abrupt in onset and progressive. She had a history of occupational exposure to livestock (sheep) and denied any episode of chickenpox in the past. She was a recently diagnosed case of peripheral T-cell lymphoma-not otherwise specified (CD-30+) and had completed one cycle of chemotherapy [Rituximab, Cyclophosphamide, Hydroxydaunorubicin, Oncovin, and Prednisone (R-CHOP) regimen]. Based on history and clinical examination, she was diagnosed as a case of varicella and started on Injection Acyclovir 500 mg IV every 8 hours for 7 days with isolation. However, the lesions continued to increase in size and number, with multiple fresh crops appearing daily, hence she was referred back after 7 days. On review, multiple hemorrhagic vesicles and flaccid bullae with thick necrotic crusting and umbilication were seen over the face, trunk, and extremities, with a few demonstrating clusters of a ring of pearls and targetoid appearance (Figure 1). Palms, soles, lips, and nasal mucosae were involved. All other mucosal surfaces were unremarkable. Small, non-tender, mobile cervical and axillary lymph nodes were noted. Differential diagnoses of hemorrhagic varicella, disseminated herpes simplex, and severe HFMD were considered.
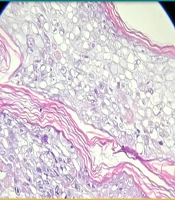
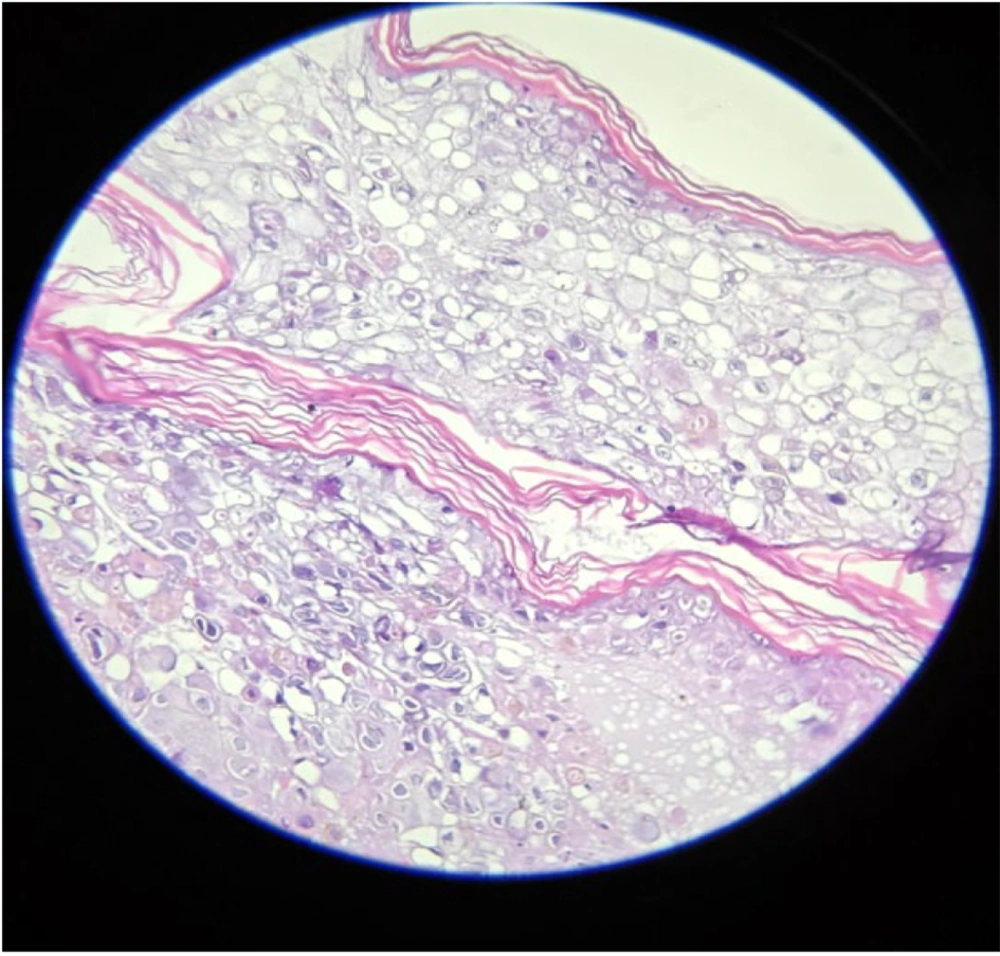
Complete blood count revealed severe anemia (hemoglobin 6.8 mg/dL) with hypoalbuminemia (2.46 g/dL); other laboratory and radiological investigations were unremarkable. A Tzanck smear demonstrated multinucleated giant cells (MNGCs). Histopathological examination revealed an intraepidermal vesicle filled with balloon cells (large cells with centrally situated nuclei, at places multinucleated, surrounded by a clear halo). The superficial and deep dermis showed periadnexal and perivascular lymphocytic infiltrate with congested blood vessels (Figure 2). Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) on a vesicular swab was positive for pan-enterovirus, leading to a final diagnosis of HFMD. She was continued on Tab Acyclovir 800 mg 5 times per day for 2 weeks (until crusting of all lesions) and topical antibiotics. Chemotherapy was given as scheduled.
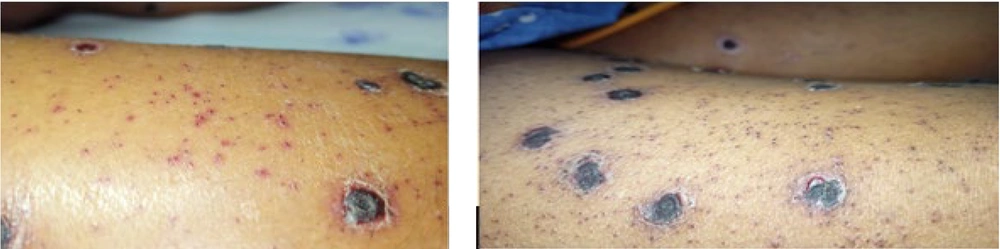
At two weeks post-treatment, all the lesions developed adherent ecthyma-like black crusts with a few partially blanchable purpura-like macules. There was no appearance of new crops, and eventually, all lesions started resolving (Figure 3). A few days later, the patient developed sudden-onset dyspnea and was started on respiratory support. Sputum culture grew Acinetobacter baumannii, for which appropriate antibiotics were given. Unfortunately, she succumbed to hospital-acquired pneumonia 6 weeks after contracting the HFMD infection.
3. Discussion
The HFMD is a common viral illness among children, infrequently affecting adults. However, there is an increasing number of cases being reported in adults with both typical and atypical presentations (1). Various outbreaks have been reported worldwide, dating back to 1957. The first HFMD case from India was documented in Calicut (Kerala) in 2005; subsequently, 81 cases were detected (2). The HFMD is caused by various serotypes of the enterovirus group, the most frequently encountered being Coxsackie virus A16 (CVA16) and Human enterovirus 71 (HEV71). Others include Coxsackie virus A (CVA) 4, 5, 9, 10, and Coxsackie virus B (CVB) 2, 5 (3). Both EV71 and CVA16 belong to the genus Enterovirus in the family Picornaviridae (4, 5), which was isolated in the cutaneous lesions of our patient. Clinically, infection with both of these viruses manifests in similar patterns. Late spring and early summer are favorable for the infection, as seen in our case (6). Common routes of transmission are fecal-oral, oral-oral, and droplet infection. Vertical spread from mother to fetus is also reported. The disease is highly contagious to close contacts, with household contagiousness being higher than 80% in siblings, more so with enterovirus. The incubation period is 3 - 6 days (7). However, our patient denied any overt exposure to children or adults with a rash, and hence the source of the infection could not be identified.
Initially, the mucosa of the oral cavity and small intestine (ileum) gets infected, followed by regional lymph node involvement within a day. Viremia follows immediately, with the virus spreading to the oral cavity and skin. After a brief period of incubation, febrile prodrome, malaise, abdominal pain, vomiting, diarrhea, and anorexia occur. Enanthem presents as painful oral mucosal ulcers on the hard palate, tongue, and buccal mucosa. However, in our case, mucosae (except lips and nasal) were either spared or the involvement was transient and inconspicuous. Characteristically, exanthem presents as a symmetrical erythematous 2–8 mm maculopapular rash on the hands and feet, which becomes vesicular with a typical erythematous halo. Oral lesions are usually very painful, leading to decreased food intake. These resolve within a week. The sides of the fingers and dorsum of the hands are frequently involved. The vesicles are typically elliptical and sometimes painful, with the long axes parallel to skin lines. Crust formation occurs within a week and usually heals without scarring. In contrast, in our patient, the lesions were in the form of large crops of hemorrhagic vesicles and bullae with necrotic crusting and umbilication, with a few showing clusters of a ring of pearls and targetoid appearance, an extremely unusual morphology for HFMD. Most often, diagnosis is made by classical clinical presentation. Differential diagnosis includes various infectious (varicella, herpes simplex) and non-infectious dermatoses (erythema multiforme). Sometimes, paired viral serology, viral cultures from stools, and PCR can be helpful (8).
Despite its sudden onset and rapid progression, most cases resolve spontaneously within 1 - 2 weeks in the presence of an intact innate immune response (9). However, in our case, the course was prolonged, probably attributable to her immunocompromised state (lymphoma on chemotherapy). Although systemic complications are rare, myocarditis, pneumonia, and meningoencephalitis have been reported (4). Occasionally, diarrhea and arthralgia can occur. Infection in early pregnancy can lead to poor obstetric outcomes. Though subtype EV71 can additionally cause severe neurological diseases, such as aseptic meningitis and acute flaccid paralysis, with fatal outcomes (2), our patient did not experience these complications. Mortality is rare. In our case, the cause of death seems to be unrelated to the enteroviral infection, as she succumbed to bacterial pneumonia confirmed by the growth of Acinetobacter baumannii on sputum culture. The treatment of HFMD involves alleviation of symptoms, including maintenance of adequate hydration, local applications like viscous lidocaine or diphenhydramine for oral lesions, and antipyretics (10). Systemic Acyclovir 10 mg/kg/dose, every 5 hours for 5 days, may be given, especially in those with obtunded immunity or severe morbidity (11). The present case was administered an extended course of acyclovir until all the vesiculo-bullous lesions resolved with crusting. Pleconaril, an inhibitor of picornavirus capsid protein function, might be worth using in serious neurological complications of HFMD (12). Rupintrivir (AG7088), a protease inhibitor, has been found to be effective (13). Isolation for about 2 - 3 days after the onset is recommended. Certain vaccines (3-dose regimen of EV71C4a) have also been developed, with protection lasting for 2 years (14), and can be worth administering in immunocompromised individuals. The unusual features of our case include adult age, florid atypical morphology, and a protracted course.
3.1. Conclusions
The HFMD, though rare in adults, warrants its inclusion in the differential diagnosis of sudden-onset atypical vesiculobullous lesions in immunocompromised patients. Management can be challenging due to atypical presentations and a variable, protracted course, necessitating extended treatment and follow-up.