1. Background
Facial skin mesotherapy is an anti-aging mild intervention method, which has been proven to be adequately effective; therefore, it has been established worldwide among the most popular cosmetic treatments (1). Various mesotherapeutic formulations are used in everyday aesthetic medical practice. Most of them, containing hyaluronic acid (HA) molecules, offer remarkable revitalization to dull and dry skin, probably exploiting HA’s unique hydrophilic properties. To maximize this, many HA mesotherapy products have been developed (2). A newly developed mesotherapeutic formulation with hyaluronic acid now incorporates an advanced form of cross-linked monophasic mono-densified HA spheres, engineered through the SCEDIS-MSCS technology method. This technology optimizes HA’s molecular structure by creating stable, uniformly monodensified spheres that enhance tissue integration and prolong hydration effects, distinguishing it from traditional HA formulations that lack such cross-linking precision. This innovative approach of cross-linked HA spheres from native HA molecules allows deeper penetration into the dermis and prolonged tissue water retention while promoting neocollagenesis for a dual mechanism of action.
2. Objectives
Given the novelty of SCEDIS-MSCS technology, this pilot study aimed to establish preliminary efficacy and safety in a controlled setting, using a single-arm design to focus on the product’s immediate impact on skin rejuvenation, with plans for a larger RCT to confirm findings. According to the investigators, this product, although not being a wrinkle filler, seems to dramatically improve the overall skin appearance and texture by both attracting and binding a sufficient quantity of tissue water into the dermis for a prolonged time and by promoting skin collagen remodeling (neocollagenesis) as well. Considering all the above, in the present study, it was attempted to thoroughly estimate the grade of total skin improvement after being injected with the proposed HA formula.
3. Methods
3.1. VISIA System
Since detailed objectivity was the main target of the study, the VISIA system was chosen as the main tool for the post-injected skin texture improvement estimation. As far as we know, this is currently the most advanced and dependable photo imaging system. It captures detailed skin images and uses cross-polarized light to evaluate the state of the underlying skin layers (3). VISIA quantifies parameters including pore count, texture roughness, wrinkles, and pigmentation using standardized multi-spectral imaging, ensuring reproducibility.
3.2. Patients
Twenty female patients, Caucasian (mean age 45 years old, range 38-55, with a standard deviation (SD) of ± 8.30 years), Fitzpatrick type III-IV, with “thick” oily facial skin (which is universally known to be remarkably “resistant” to commonly used skin mesotherapeutic agents) were enrolled and treated with multiple superficial injections in just one session. Each subject underwent a VISIA baseline documentation before treatment and a second one 2 months later. No additional cosmetics (creams, serums, etc.) were allowed to be used during these two months. Participants with connective tissue diseases (such as lupus erythematosus), blood disorders, or cancer were not eligible for inclusion. Moreover, the intake of retinoids, salicylic acid, vitamin E, Ginkgo Biloba, and medications with anti-inflammatory or anti-platelet properties was strictly avoided for seven days before undergoing HA mesotherapy injections. Furthermore, the study did not include participants who had silicone implants in the targeted treatment area, had undergone filler procedures within the past year, or were prone to developing keloid or hypertrophic scarring. This study was performed following ethical standards outlined in the Declaration of Helsinki and Good Clinical Practice guidelines, while also complying with relevant Greek legislation. The protocol for the study, including patient participation, was officially reviewed and approved by the Local Ethics Committee of Tzaneio General Hospital in Piraeus, Greece.
3.3. Subjective Feeling of Facial Skin Upgrading
The Mesotherapy Hyaluronic Acid Skin Booster Satisfaction Scale (MHASBSS) questionnaire was also utilized to gauge patients' personal perceptions of the treatment results, with each participant providing responses at three different time points: Before treatment, just after, and two months later. The patient-tailored questionnaire was concluded by taking into account a vast retrospective study about mesotherapy-treated skin outcomes (4).
3.4. Content of the Mesotherapy Hyaluronic Acid Skin Booster Satisfaction Scales and Checklists
3.4.1. Mesotherapy Hyaluronic Acid Skin Booster Satisfaction Scale
Please rate your satisfaction with the following aspects of your mesotherapy treatment with the hyaluronic acid skin booster. The evaluation was based on a five-point scale: 1 (Very dissatisfied), 2 (dissatisfied), 3 (neutral), 4 (satisfied), and 5 (very satisfied).
(1) Overall improvement in the appearance of your facial skin
(2) Reduction in the appearance of fine lines and wrinkles
(3) Improvement in skin texture and smoothness
(4) Enhancement of skin firmness and elasticity
(5) Increase in skin hydration and radiance
(6) Reduction in the appearance of pores
(7) Evenness of skin tone and complexion
(8) Overall healthier and more youthful appearance of your skin
(9) Likelihood of continuing with this mesotherapy treatment as part of your skincare routine
(10) Likelihood of recommending this mesotherapy treatment to others seeking to improve their facial skin appearance
Scoring:
- Add up the scores for all 10 questions.
- Minimum score: 10
- Maximum score: 50
- Elevated scores signify increased satisfaction with the effectiveness of the mesotherapy hyaluronic acid skin booster treatment for facial skin enhancement.
3.5. Statistical Analysis
VISIA findings focused on the most bothersome skin surface characteristic: The skin pores. Skin texture change in general was also attended to. Results were estimated using a one-way Friedman ANOVA to assess changes across multiple time points, followed by the Wilcoxon signed-rank test for pairwise comparisons between baseline and two-month post-treatment measurements. A significance level of α = 0.05 was used unless otherwise stated (e.g., α = 0.10 for skin texture). P-values were interpreted alongside effect sizes (e.g., percentage changes) to assess clinical relevance. All analyses were performed using SPSS version 26. The MHASBSS questionnaire was statistically estimated by proper bar charts according to the established methods (4). The MHASBSS questionnaire data were analyzed using paired t-tests to compare mean scores at different time points (before, immediately after, and two months post-treatment). Detailed statistical outputs, including mean values, standard deviations, and confidence intervals, are provided in Appendix 1 in Supplementary File.
3.6. Mesotherapeutic Agent
The injection of the under-study HA product was performed into the papillary dermis using a 32G needle with a 4 mm length, according to each participating physician’s decision. The total amount of the product used did not exceed 4 ml per face. Further analyzed, this innovative HA product consisted of cross-linked HA spheres, thoroughly mixed with native HA molecules (“swimming into a pool of HA”). This type of unique mixing became feasible due to SCEDIS™–MSCS technology and enhanced deeper penetration of the product into the upper and mid dermis. The viscoelastic properties of the injected material, along with the abundant recruitment of tissue water, were anticipated to result in remarkable skin texture rejuvenation, which was finally assessed and rated.
4. Results
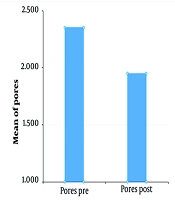
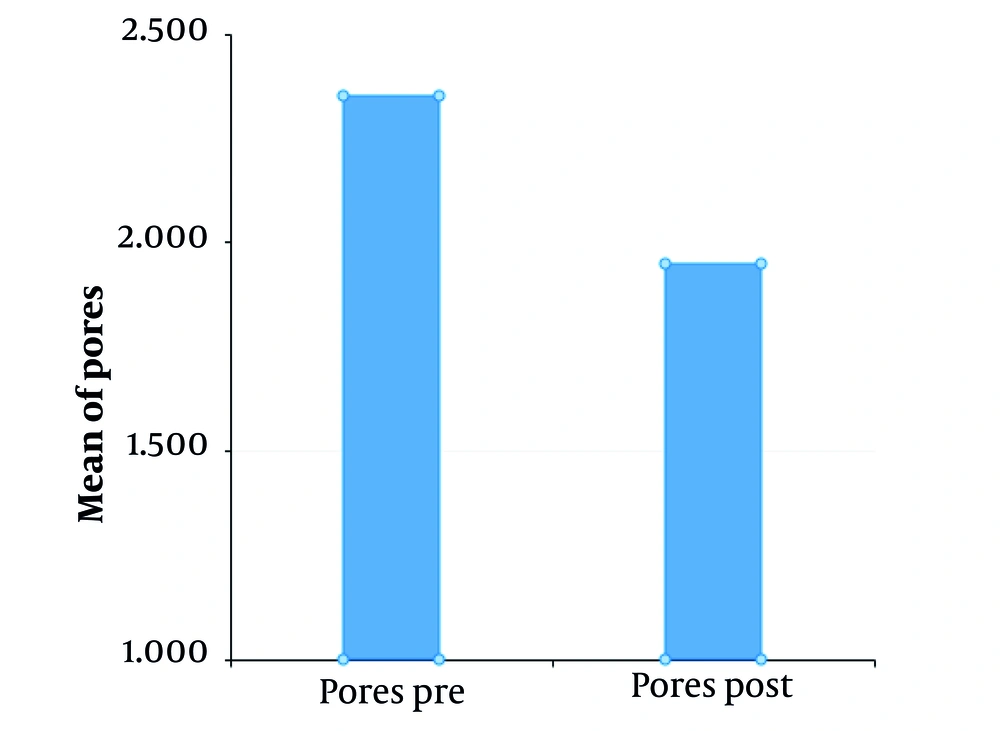
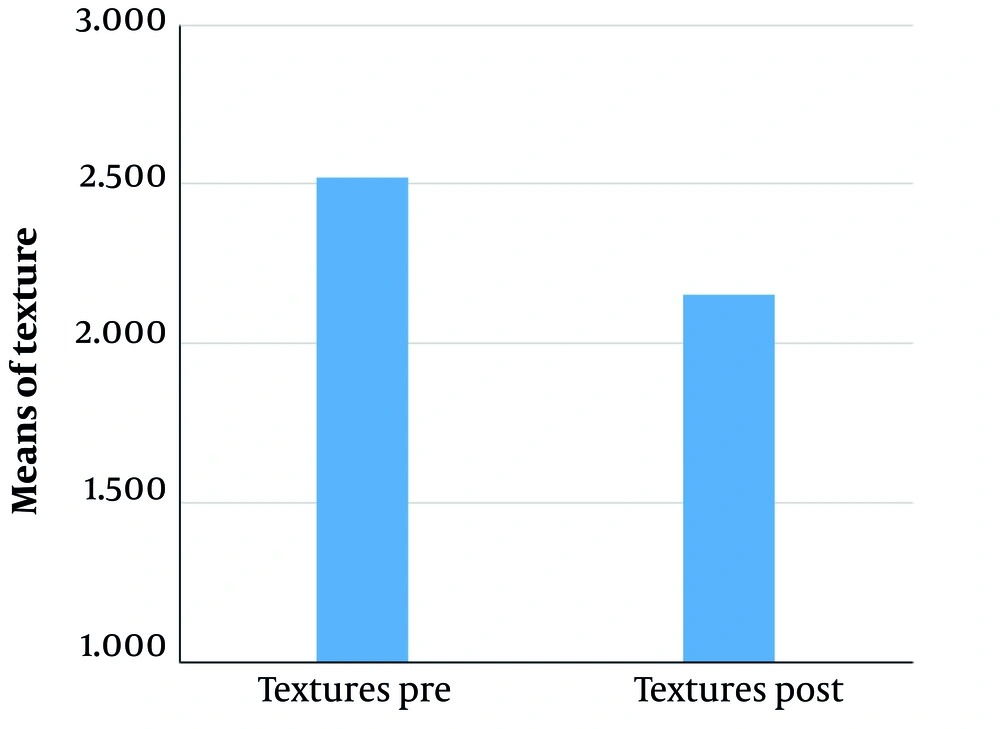
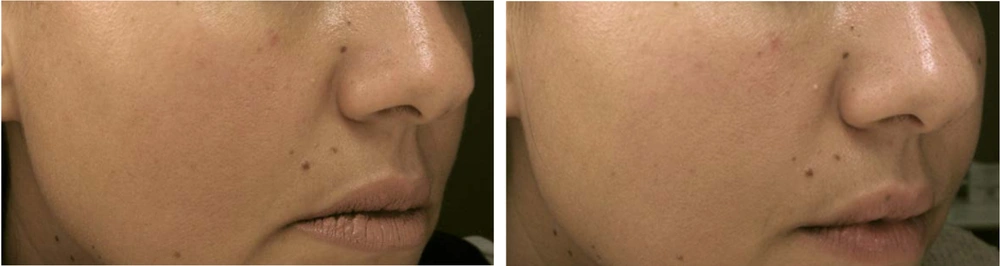
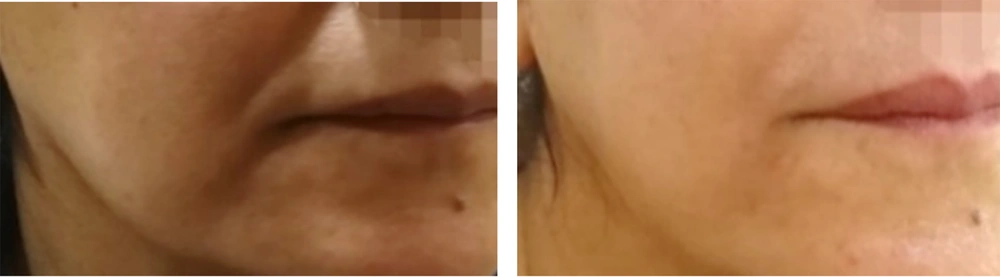
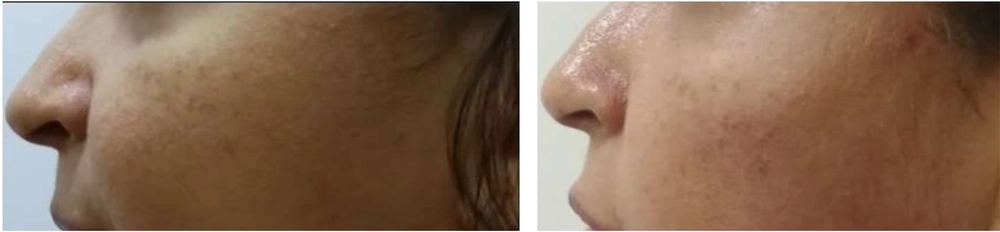
VISIA counting bars, as studied by the Wilcoxon signed-rank test, documented a statistically significant improvement for the variables of skin texture (P = 0.05 for α = 0.10***, mean roughness reduction of 12.8%, SD ± 6.5 ***) and pores (P < 0.01 for α = 0.05 ***, mean pore count reduction of 15.3%, SD ± 7.2 ***), before and two months after treatment, as shown in Figures 1 and 2. Additional VISIA parameters showed a 10.2% reduction in wrinkle severity (SD ± 5.8, P = 0.08) and a 9.5% improvement in pigmentation evenness (SD ± 4.9, P = 0.10), though these were not statistically significant. Characteristic results are also depicted in Figures 3, 4, and 5. Figures 3, 4, and 5: Photos taken before and two months after just one single mesotherapy treatment with the innovative hyaluronic acid injectable agent. Each photo depicts a different study participant. Remarkable skin refinement is clearly shown. Simultaneously, skin pores are visibly reduced in both size and number. The VISIA findings coincide with obvious facial skin rejuvenation.
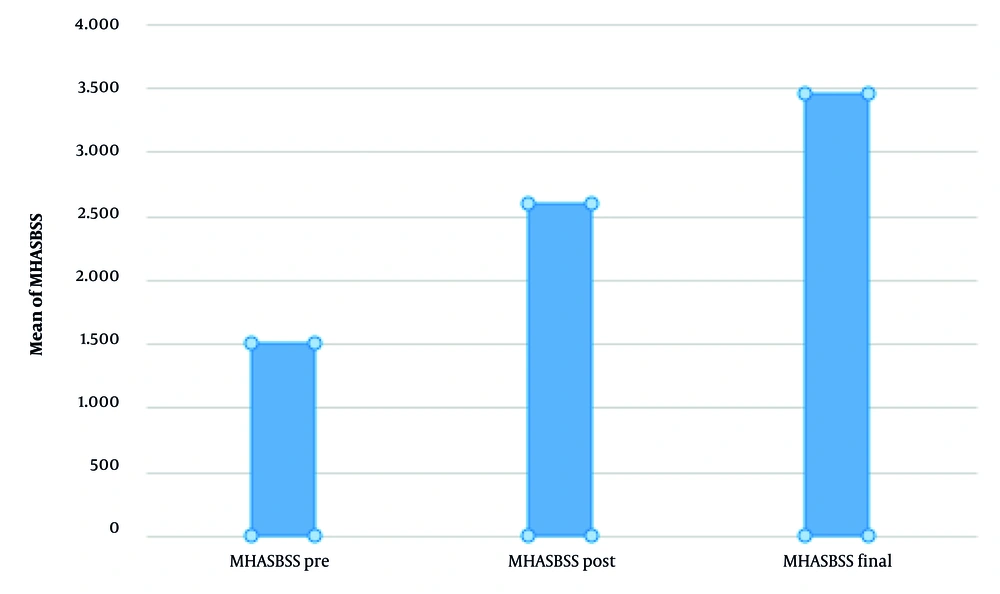
As expected, the MHASBSS questionnaire (as seen in Figure 6), provided by the patients, offered results remarkably favoring the skin-boosting effect, with P-values: 0.05 for comparison before and after mesotherapy treatment, 0.05 for the satisfaction feeling just after the treatment session and two months later, while the P-value for the participants' feeling before and two months after treatment is equal to 0.001. Mean MHASBSS scores increased from 28.5 (SD ± 4.2) before treatment to 42.3 (SD ± 3.8) two months post-treatment, reflecting high patient satisfaction. Appendix 1 in Supplementary File provides detailed mean values, standard deviations, and 95% confidence intervals for VISIA measurements and MHASBSS scores.
5. Discussion
There are two ways to rate the outcome of aesthetic interventions: The objective method and the subjective one, which considers the treated person’s feeling about the overall result (5). In the present study, VISIA analysis offered the objective tool, while the MHASBSS questionnaire provided the subjective one. As shown above, the total effect remarkably favored the usage of the studied HA molecule as a mesotherapeutic agent. This could be attributed to skin rejuvenation by injected HA components, as it has already been broadly evidenced by Jeon 2020, Bravo 2022, Keen 2017, and Salwowska 2016, demonstrating HA’s role in hydration and neocollagenesis (4, 6-8). The VISIA observation as well as the MHASBSS answering registration were concluded two months after the injections, emphasizing whether the cosmetic result was just temporary or prolonged, since it has been noted that although remarkable refinement of the skin usually follows mesotherapy, in a lot of cases it fades within the first couple of weeks (9). As it was clearly noted, two months after mesotherapy injections, the HA molecule under investigation was shown to consistently maintain a prolonged, reliable cosmetic result. This was probably due to the innovative HA molecule’s ability to maintain a proper humid environment, promote various growth factors stimulation, and offer the necessary scaffold for cellular recruitment and adhesion, advancing overall dermal refreshment.
This study has limitations that contextualize its findings. The small sample size (n = 20) and focus on Caucasian females with Fitzpatrick type III-IV oily skin limit generalizability to broader populations. The absence of a control group, such as a placebo or alternative HA product, prevents definitive conclusions about the specific efficacy of the SCEDIS-MSCS HA formula relative to other treatments. The two-month follow-up may not capture long-term outcomes, as HA effects may wane or strengthen over time. Additionally, the VISIA system, while robust for surface metrics, may not detect deeper dermal changes (e.g., collagen density). These limitations are common in pilot studies, which prioritize feasibility and initial efficacy (9).
To address these, future studies should include randomized controlled trials with placebo or comparator arms, larger and more diverse populations (including males and varied skin types), longer follow-up periods (6 - 12 months), and histological analyses to confirm neocollagenesis. Future research needs to build on these preliminary findings; a follow-up multicenter RCT is planned, incorporating a placebo control group (saline injections) and an active comparator (a standard HA mesotherapy product). The study will target a sample size of 100 participants (50% male, diverse skin types, Fitzpatrick I–VI), calculated to achieve 90% power to detect a 10% difference in texture improvement (α = 0.05). Follow-up assessments at 6 and 12 months will evaluate durability, with additional endpoints including skin hydration (via corneometry) and collagen density (via biopsy). This design will address the current study’s limitations, providing robust evidence for the SCEDIS-MSCS HA product’s efficacy and positioning it within the broader mesotherapy landscape.
Clinically, this HA product offers a non-invasive option for skin rejuvenation with minimal downtime, potentially benefiting aesthetic practices. The novel HA skin booster, utilizing SCEDIS-MSCS technology, significantly improved skin texture, pore size, and patient satisfaction, as evidenced by VISIA and MHASBSS data in this pilot study. These findings highlight its potential as an effective mesotherapy agent, with plans for a larger RCT to confirm and extend these results.