1. Background
In recent years, soft tissue augmentation techniques have gained much public acceptance, due to increased patients’ tendency to improve their appearance without undergoing a major surgery. Several types of fillers are used to improve soft tissue structure and appearance. Although tissue augmentation by fillers has many advantages over surgery, transient nature and temporary effect of most fillers makes repeated treatments inevitable. More than 30 different types of fillers have been qualified for use in Europe; however, food and drug administration (FDA) has approved few of them, including autologous fat (AF) for use in the United States (1).
Injecting AF filler is one of the safest and the most ideal filler for most people. Its benefits include absence of hypersensitivity reactions, abundant source of fat and possibility of creating permanent effect (2). AF is commonly used to fill depressed areas of the skin. It is estimated that around 15% to 20% of injected fat has the ability to survive and be effective; therefore, to achieve favorable results, patient requires three to four injections (3).
Autologous platelet gel (APG) produced from platelet-rich plasma (PRP) increases survival of lipocytes and lipoblasts. Several types of anabolic and trophic growth factor has been identified in platelet gel, most notably, platelet-derived growth factor (PDGF) and transforming growth factor β1 (TGF-β1). Other important proteins that participate in the healing of ulcers include epithelial growth factor (EGF) and insulin-like growth factor (IGF), which are secreted from activated platelet granules. These growth factors (GFs) can increase collagen deposition, angiogenesis, proliferation of fibroblasts and extracellular matrix synthesis, all of which are associated with the healing process and reconstruction of soft tissue (4-8).
Several studies have been performed on the effects of GFs on fat cells. Various studies have shown that adding GFs can improve the outcomes of AF injection in restoring depleted tissues (9).
The key factor in the process of tissue regeneration is using an ideal material able to provide both structural support and appropriate environment for cell growth. In a study, using autologous PRP as a reservoir for fat cells increased survival of fat cells and stimulated angiogenesis (10). In another study, fibrin was used as a carrier for fat precursor cells (pre-adipocytes) and resulted in stable fat cells with long-term survival (11). Several studies have been performed on the effect of GFs in various medical fields and have shown the effectiveness of adding GFs in wound healing (12), especially in trauma and orthopedics (13). In addition, injecting fibroblast-derived GFs in combination with AF cells was effective in repairing vocal cords affected by tuberculosis (14). No detailed studies concerning the combination of AF and APG (AF-APG) in nasolabial fold augmentation has been performed yet.
2. Objectives
In this report, the effectiveness of AF injection in nasolabial folds augmentation was compared with AF-PG injection alone.
3. Patients and Methods
3.1. Patients
Nine subjects (three males and six females) aged 55 to 72 years old with deep nasolabial folds were treated using this new technique. Exclusion criteria were autoimmune diseases such as systemic lupus erythematous, systemic sclerosis, sarcoidosis, immunodeficiency due to disease, medicine or chronic infectious diseases such as infection with human immunodeficiency virus (HIV) and hepatitis. The study protocol and method of therapy were explained to all participants. Blood coagulation tests, complete blood count and HIV and hepatitis-screening tests were performed before enrollment. Then a written consent form, containing details of study and patients right was signed by each participant.
3.2. Preparation of Platelet Gel
First, 10 mL blood was taken from each participant in blood transfusion service. Then 5 mL APG containing GFs (including fibroblast growth factor (FGF), EGF and PDGF) and coagulating factors such as fibrin was produced. On the day of operation, APG was transferred to the hospital.
3.3. Surgical Procedure
Under local anesthesia and aseptic conditions, tumescent (1% lidocaine, 1/100000 epinephrine and Ringer’s lactate) was injected subcutaneously in lateral lumbar region. Twenty minutes later, liposuction was performed using the liposuction cannula (14 gauge 10 cm long) attached to a 10-mL syringe. After washing the fluid with normal saline for several times, 8 mL of relatively pure fat was obtained for one site of treatment. Then using another 10 mL syringe and with the same method, 4 mL of relatively pure fat was collected that was mixed by 4 mL APG for the other site. After liposuction, surgical site was washed and covered by a sterile pressure dressing.
After warming APG, 4 mL gel was added to a syringe containing 4 mL fat, resulting in an 8 mL AF-PG. After providing local anesthesia by injecting 2% lidocaine, one side of the face was randomly treated with AF and the other side with AF-PG.
3.4. Clinical Assessment
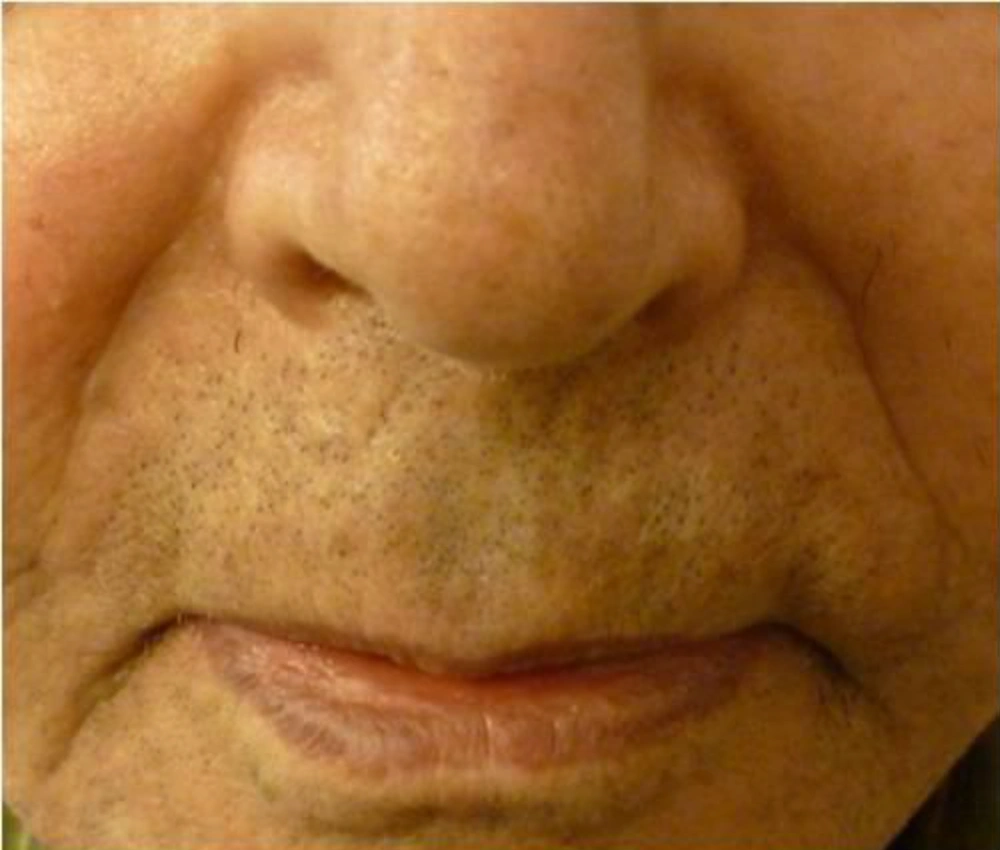
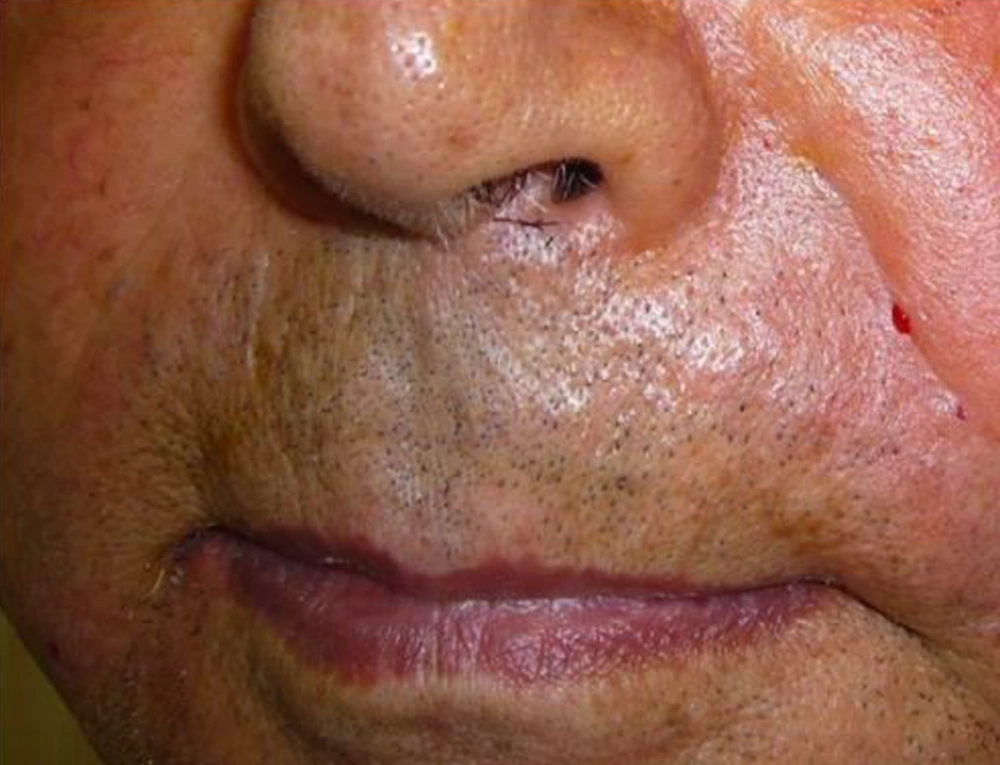
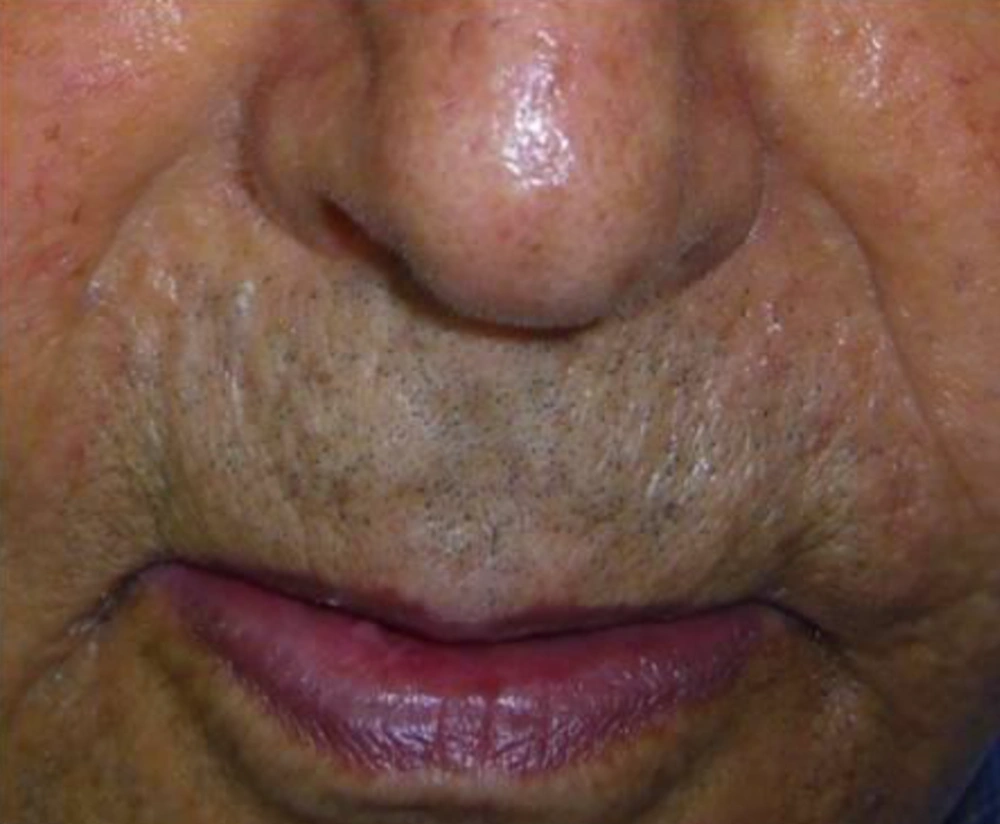
The nasolabial folds were photographed before injection and monthly for the next six months with digital camera (Nicon, COOLPIX S1000, Japan). Based on visual analog score (VAS), improvement was categorized into four grades as follows;
1. Grade I: very good fold and very good cosmetic outcomes (depth of the fold range, 1% - 25%).
2. Grade II: good fold and desirable cosmetic outcomes (ranged 26% - 50%).
3. Grade III: medium fold and relatively good cosmetic outcomes (ranged 51% - 75%).
4. Grade IV: profound fold and adverse cosmetic outcomes (ranged 76% - 100%).
Intervention improvement was evaluated by two independent dermatologists and patients were unaware of the protocol of treatment. The grade of fold was recorded at baseline, immediately, one, three and six months after the treatment.
3.5. Side effect
Patients were asked about side effects including edema, erythema, ecchymosis and tenderness during two weeks after injection on both sides one month later.
4. Results
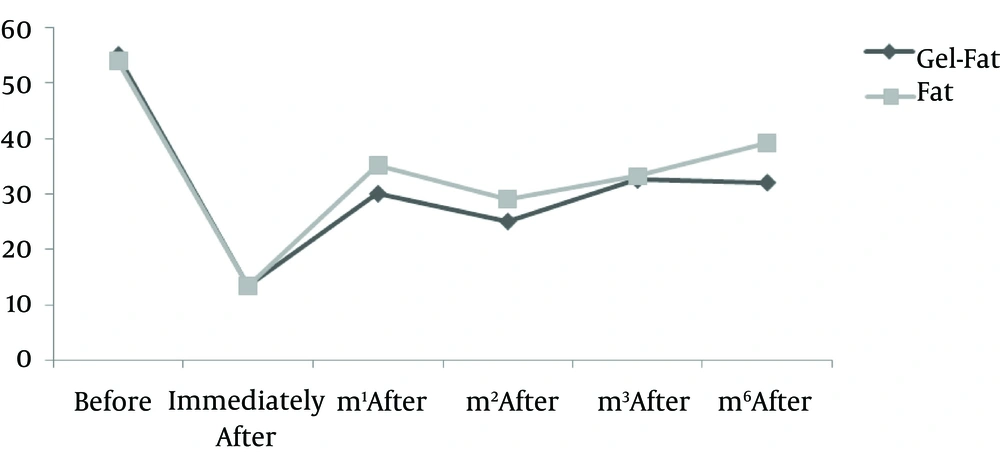
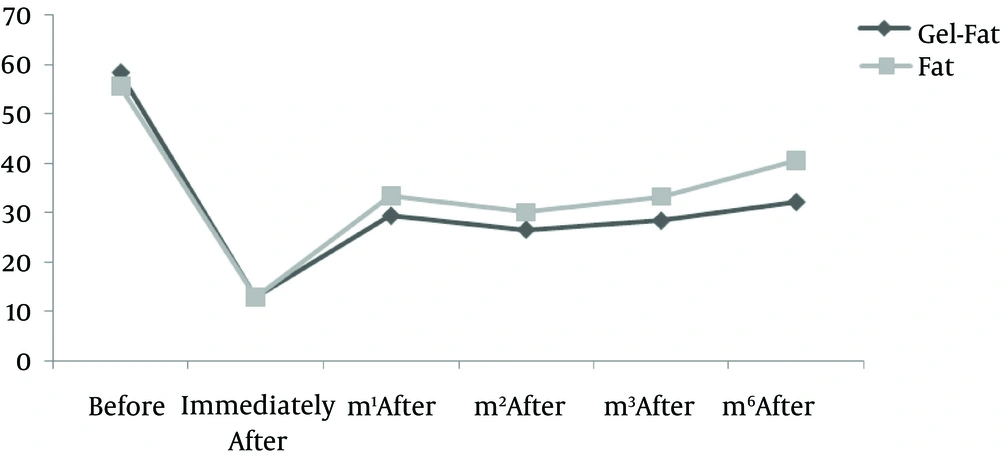
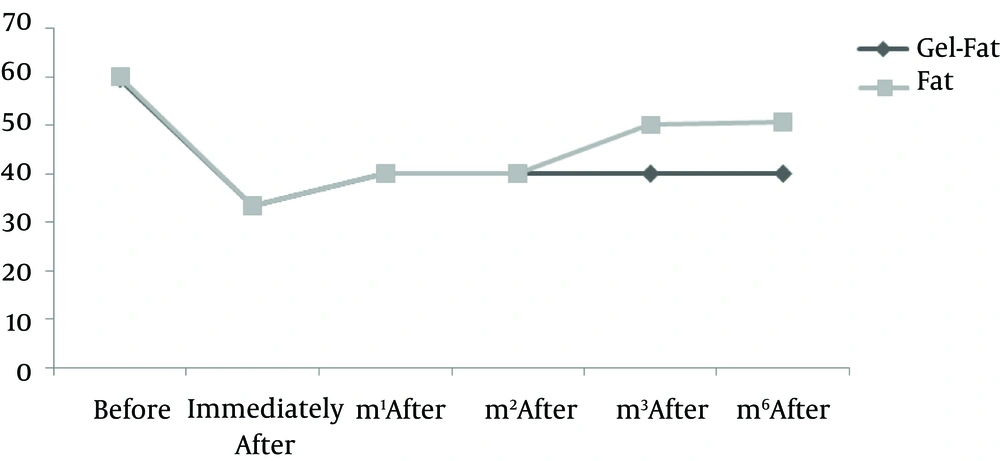
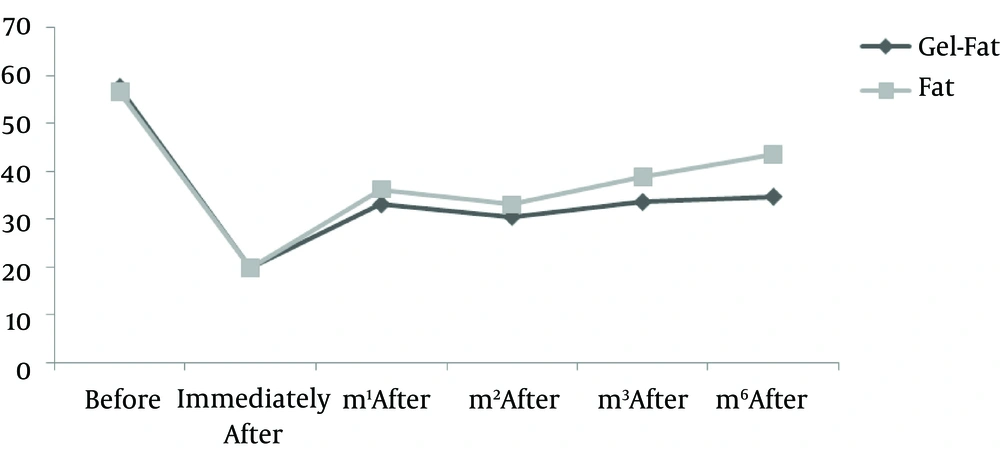
Nine patients including three males and six females were enrolled in the study. Mean age of participants was 61.2 years old (55 - 72 years). All participants had bilateral deep nasolabial folds and each side was treated randomly by fat or gel-fat injection technique. Assessment of deficit in folds after treatment was performed immediately after injection and in one, two, three and six months later by two independent dermatologists and patients themselves.
Results showed significantly decrease of nasolabial fold deficit in both of therapeutic methods immediately after treatment and no difference between them.
Whereas at follow-ups, fold deficit increased gradually but improvement was always more in gel-fat group in the first, second, third and sixth months after the treatment. Differences except for the second month were statistically significant (P: 0.02, 0.16, 0.003 and > 0.001, accordingly). Mean of deficit assessments based on examiner are shown in Figures 1 - 4.
Complications of treatment included tenderness, erythema, ecchymosis and edema shown in Table 1.
5. Discussion
In this study, the effectiveness of AF-APG on nasolabial augmentation was assessed and compared with a single injection of AF alone. Although only nine patients were treated with this method, considerable and significant results were obtained. After one month in all patients, a greater improvement was seen in the side treated with injection of APG and fat than the side treated with AF only. This improvement was more pronounced in the third month and at the end of sixth month after the treatment. After six months of injections, the degree of wrinkles on side of AF-PG therapy was less than that of the other side in all subjects. Interestingly, this difference was clearly significant according to the evaluation by both specialists as well as patients. Along with superior effectiveness of AF-PG, lowest complication in this treatment was also important; however, in the side treated with AF alone five cases of ecchymosis were reported. The absence of ecchymosis in AF-PG therapy could be due to coagulating effects of APG.
Soft-tissue fillers were used for the first time in 1980. At the time, Neuberhad used the blocks of free fatty, drawn from individual’s arm, to repair depressed areas of the skin. The injected fat loses 50% of its size over only a year in most cases and substantial progress in the process of injecting the fat had not been happened; nonetheless, using soft-tissue fillers has been increased rapidly over recent decades. For example, in studies by the american society for dermatologic surgery (ASDS), injection of soft-tissue fillers was among the five most common practices of cosmetic surgeons and increased by 30% between 2005 to 2007 (15). Although the history of soft tissue augmentation dates back to over a century ago, fillers injection for cosmetic surgery using bovine collagen was greatly evolved in 1980s (16).
During recent years, new and more effective compounds for removing wrinkles have been evaluated. For example, Baumann et al. examined the efficacy of three different types of skin fillers containing hyaluronic acid gel with cross-linked collagen in the treatment of nasolabial wrinkles in 2007. In this randomized double-blind clinical trial including 439 patients, three types of gel containing hyaluronic acid, namely J30, 24HV and 30HV and Zyplast cross-linked collagen were used to treat patients (17). According to their results, all three types of hyaluronic acid-containing gels led to more prolonged modification in comparison with collagen. In follow-up of over 24 weeks after the last treatment, 90%, 88% and 81% of patients receiving respectively 30HV, 24HV and J30 gels showed a significant therapeutic advance. While in the group treated with collagen, therapeutic effects were shorter and at the end of 24 weeks, only 36% to 45% of therapeutic effects had remained. In addition, the only complication of hyaluronic acid treatment in this study was mild to moderate reactions at the injection site (17).
The effect of AF-PG in repair of wrinkles has not been studied so far. The effects of APG on healing of acute and chronic wounds were assessed in only few studies with mostly reporting favorable results. In one of the first studies, Hom et al. studied the effects of APG therapy in the treatment of acute cutaneous wounds (12). In their prospective clinical trial, 80 full thickness skin wounds (4 mm diameter) in the thigh of eight healthy individuals under treatment with APG or antibiotic ointment were monitored and photographed during six months. Moreover, the tissue samples were used to evaluate and compare histological changes in healing wounds. The results of this study showed that after 42 days, more noticeable and appropriate improvement occurred in the wounds treated with APG. Therefore, in the 17th day, 80% of wounds closed in APG group, while closure was seen in less than 60% of wounds in the other group. Overall closure rate in the group treated with APG was significantly higher than controls (P = 0.001). Microscopic examination suggested that process of epithelialization and granulation in the APG group was on average three days shorter than in the control group and proliferation of endothelial cells was increased in the former group (P < 0.04).
The results of the study by Hom et al. showed that AF-PG had more effect on healing process of chronic wounds than that of acute wounds (12). In a study conducted by Steed et al. on ulcer of chronic neurotrophic diabetic foot, PDGF with appropriate care had a favorable effect on healing chronic wounds (18).
Several theories have been developed with respect to the mechanism of APG effect on wound healing or improvement of skin wrinkles. Regarding healing of acute wounds, it is discussed that granulation tissue formation and epithelialization would increase with APG; however, it has been observed when the platelet concentration in the gel was about six times as high as its concentration in the plasma (12). Another theory concerns the contractile effect following APG injection that develops at the site of wound and causes accelerated wound closure. According to some studies, PDGF and TGF-β1 found abundantly in the APG cause fibroblasts transformation into myofibroblast and can lead to myofibroblasts contraction (19, 20). Moreover, high calcium concentration, which is used in APG preparation is effective in induction of wounds and skin defects contraction (12). Regarding chronic wounds, the concentration of FGF and TGF-β would be reduced following down regulation of related gene in wound and healing process would be accelerated following APG injection.
Limitations of this study were its small sample size and VAS assessment of improvement.
APG plays a role in the healing of skin wrinkles; however, determining its mechanism requires more complementary studies. Meanwhile, the use of APG reduces possible complications of AF injection such as bleeding and hematoma.