1. Background
Vitiligo is an acquired, chronic, idiopathic disease characterized by circumscribed depigmented macules (1) and patches that might enlarge slowly. Clinically, vitiligo is classified into localized, generalized and universalis. Vitiligo involves 1% of the population and is seen equally in all races and in both genders (2). It is a multifactorial polygenic disorder, although various hypotheses have been proposed including autoimmunity, viral infection, biochemical, neural and oxidant/antioxidant theories (oxygen radical species), yet the exact mechanism of the disease has not been established yet (3).
Eyes are one of the most important organs that might be involved in patients with vitiligo (4). Recent studies have indicated a strong association between vitiligo and ocular findings (4). Vitiligo can involve the appearance of eyes inducing depigmentation of eyelids and poliosis (4, 5). It is well known that the uveal tract and retinal pigment epithelium contain pigment cells and association of vitiligo with inflammation of the uveal tract has long been recognized, primarily in the form of uveitis, as seen in Vogt-Koyanagi-Harada and Alezzandrini syndromes (6, 7).
2. Objectives
To evaluate the prevalence of ocular findings in patients with vitiligo and reveal any risk factors or possible associations that might increase the risk of ocular manifestations.
3. Patients and Methods
In this study, 92 patients with previously documented cutaneous vitiligo of any type were examined for ocular abnormalities from April 2007 to September 2008, at the Rassoul Akram Hospital. The demographic features of the patients including age, gender, duration of vitiligo, presence of associated autoimmune disease and its type, positive family history, as well as the anatomical distributions of depigmented macules were recorded. Vitiligo patches were distributed on the head, neck, trunk, upper and lower extremities and genitalia. Standard ocular examinations including visual acuity test, external examination, biomicroscopy and dilated fundoscopy were performed. The results were recorded in related flow sheets and were analyzed using the SPSS software version 15 by t-test and chi-square test.
4. Results
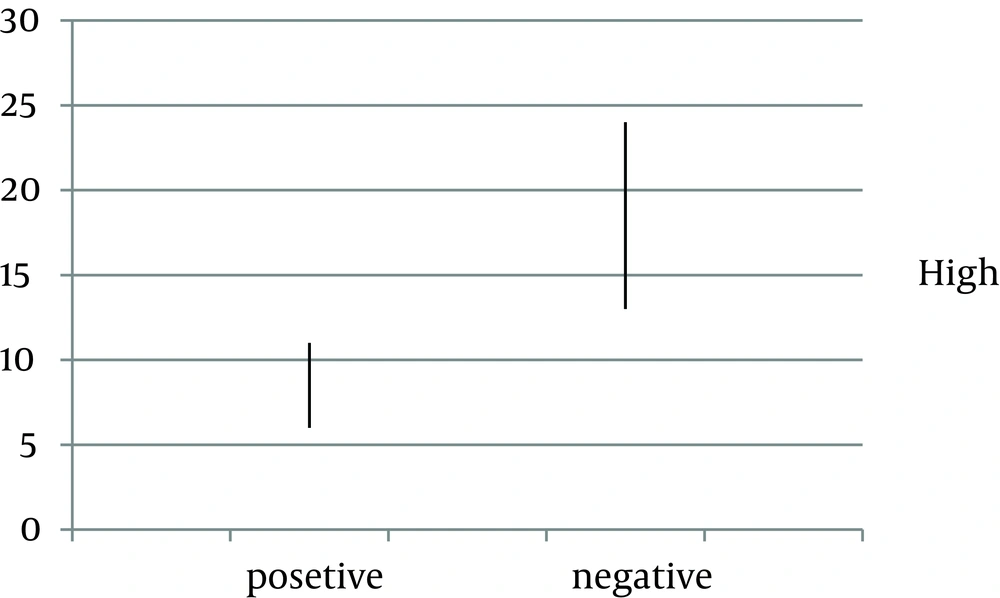
In this study, 92 patients including 47 (51.1%) male and 45 (48.9%) female, with documented Vitiligo were enrolled. The mean age of the participants was 32.1 ± 13.16 years (ranging from 9 to 65 years of age). The mean duration of disease was 10.84 ± 10.17 years. Ocular abnormalities were found in 19 (20.7%) patients including retinal hypo pigmentation in 5 (5.4%), optic atrophy in 4 (4.3%) and uveitis including any type of uveitis in 10 (19.0%) patients. In 26 (28.3%) patients, first-degree relatives had vitiligo (Table 1). Positive history of autoimmune diseases was found in 19 (17.48%) patients (Table 2) including diabetes mellitus and hypothyroidism in ten and nine patients, respectively. Depigmented macules were seen mostly on the upper extremities (60.8%); genitalia had the lowest percentage of distribution (10.8%). The mean age of patients who had ocular findings was significantly higher than those without these problems (P < 0.0001). In addition, the duration of Vitiligo was longer in patients with ocular findings (P > 0.0001) (Figure 1).
| Family History | Ophthalmic Involvement | Total | |
|---|---|---|---|
| Yes | No | ||
| Yes | 15 (57.7) | 11 (42.3) | 26 (100) |
| No | 58 (79.3) | 8 (20.7) | 66 (100) |
a Data are presented in NO. (%).
| Associated Diseases | Ophthalmic Involvement | Total | |
|---|---|---|---|
| Yes | No | ||
| Yes | 62 (84.9) | 11 (15.1) | 73 (100) |
| No | 11 (57.9) | 8 (42.1) | 19 (100) |
| Total | 73 (79.3) | 19 (20.7) | 92 (100) |
a Data are presented in NO. (%).
No statistically significant association was found between sex and ocular findings (P > 0.05, Table 3). Ocular findings in patients with positive family history were significantly more prevalent than in those without ocular problems (P = 0.001) (Table 2). No statistically significant association was found between anatomical distribution of depigmented macules and ocular abnormalities (P > 0.05). Positive history of autoimmune disorders was significantly more common in patients with positive ocular findings than in those without ocular problems. (P = 0.01)
| Gender | Ophthalmic Involvement | Total | |
|---|---|---|---|
| Yes | No | ||
| Male | 37 (78.7) | 10 (21.3) | 47 (100) |
| Female | 36 (80) | 9 (20.0) | 45 (100) |
a Data are presented in NO. (%).
5. Discussion
Destruction of melanocytes in vitiligo is caused by combination of immunological and cytotoxic mechanisms. In addition to cutaneous melanocyte, melanin-containing cells of leptomeninges, inner ear and eyes might be destroyed. With regards to eye changes, it should be recalled that two distinct populations of pigment-bearing cells exist: the uveal melanocytes and pigment epithelium (8). Destruction of uveal melanocytes and pigment epithelium in patients with vitiligo was first documented by Albert et al. (9), who reported various abnormalities in 112 patients, including uveitis, retinal pigment epithelial hypopigmentation, choroidal scars, pigment clumping and transillumination defects. In the same year, Rosenbaum et al. (10) reported a case of bilateral retinal pigment epithelial changes associated with periorbital vitiligo and seizure. Four years later, Albert et al. (9) found asymptomatic and symptomatic retinal pigment epithelium atrophy in 27% of 223 patients with vitiligo; this was the only controlled study so far that compared ocular findings in patients with vitiligo and psoriasis and demonstrated a significant increase in retinal pigment epithelium atrophy or hypopigmentation in patients with vitiligo. In a subsequent study by Cowan et al. (11) ocular inflammation was not found as a major feature in patients with vitiligo and 40% of patients showed some degree of fundal pigment findings including pigment clump, focal hypo pigmented spots, and choroidal nevi. The most common ocular findings in our study were peripapillary atrophy around the optic nerve, atrophy of retinal pigment epithelium and diffuse and focal hypopigmented spots on the retina. During 2006, a study done on 45 patients with vitiligo examined for ocular findings (5) in addition to evaluation of demographic features including age, gender, duration of vitiligo, association with autoimmune disease and anatomical distribution of depigmented macules to evaluate any probable association with ocular abnormalities. The results showed ocular abnormalities including iritis, peripapillary atrophy, pigmented epithelium atrophy and focal hypopigmented spots in ten (22.23%) patients (5). Anatomical localization, primarily periorbital, and to a lesser extent vitiligo of the genitalia were reported to be the most probable alerting features for ocular findings. Another study performed on 150 patients with vitiligo during 2007, revealed 24 (16%) cases of ocular problems including uveitis and pigment abnormalities in iris and retinas in which 18 patients had hypothyroidism, diabetes mellitus or alopecia as an autoimmune disease. The ocular findings of our study were similar to those of the literature and were not associated with any other abnormalities.
It is evident that multiple ocular abnormalities might be found in patients with vitiligo. Many ocular abnormalities are non-specific in character and distribution; however, they might be ascribed mostly to immunological processes in vitiligo. In this study, we aimed to determine any features that might be related to ocular findings. Our study revealed a significant association between age and duration of vitiligo with ocular findings and showed that positive family history was an important risk factor for patients presenting ocular problems. The prevalence of ocular findings was significantly higher in patients with autoimmune diseases. Our findings were similar to recent studies and showed that with increasing age and duration of vitiligo, the risk of ocular findings was greater. In addition, having a positive family history and autoimmune disease were important risk factors that increased ocular findings. This study showed that ocular problems are important and assessable findings in patients with vitiligo. In addition, aging and duration of vitiligo can increase the risk of ocular abnormalities and patients with a positive family history and autoimmune disease should be on priority of screening.