1. Background
Melasma is a commonly acquired hypermelanosis on sun-exposed regions and is characterized by symmetric hyperpigmentation with irregular margins that involve the cheeks, forehead, nose, upper lips, and chin, although other sun-exposed regions may also be involved (1, 2). Sun exposure is the most important predisposing factor. Several studies have proposed other predisposing factors, e.g., genetic factors, female gender, skin phototype III-V, sexual hormone levels, pregnancy, thyroid disorders, cosmetics, and drugs; however, the exact etiology remains unknown 1. Because of frequent relapses, treating melasma is difficult and frustrating. Hydroquinone has been considered as a first-line therapy as it moderately decreases pigmentation in almost all patients. Other treatment modalities include tretinoin (3-5), corticosteroids (6), kojic acid (7), azelaic acid (8, 9), and third mixture (10, 11). Intense pulsed light, lasers, and dermabrasion have been considered as adjuvant therapies (12-14). The efficacy of 5% methimazole cream has been recently reported in 2 patients with treatment-resistant melisma (15). This suppresses melanin synthesis by inhibiting peroxidase (16-22) and thyrosinase (23, 24). Unlike hydroquinone, methimazole does not lead to the death of melanocytes (18) and has no cytotoxic or mutagenic effects (25, 26).
This study aimed at evaluating the therapeutic effect of topical 5% methimazole versus 2% hydroquinone in females with melasma.
2. Methods
2.1. Study Design and Participants
This study was designed as a double-blind randomized clinical trial. Fifty-eight patients with clinically diagnosed melasma, who were referred to outpatient clinic of the current study in Tehran, between 2015 and 2016, were enrolled. Inclusion criteria included age of 18 to 50 years old, skin without comedones, and normal-to-dry skin. Exclusion criteria included pregnancy and lactation, thyroid disorder, use of oral contraceptive pills or other drugs that can induce melasma-like hyperpigmentation (anti-psychotic drugs, anti-inflammatory drugs, phenytoin etc.), use of topical or oral whitening drugs in the previous month, and history of whitening procedures in the previous 6 months.
2.2. Procedure and Assessment Variables
Before initiating the treatment, all patients provided written informed consent, and the study was approved by the ethics committee of the Iran University of Medical Sciences (Tehran, Iran). Detailed histories and standard digital photographs (with a Canon EOS 1000D camera, 14 megapixels, and a VisioFace 1000 D, KhazakaElectronic, Germany) were obtained. Melasma area and severity index (MASI) scores were calculated for all patients before and after the treatment. To verify the safety of methimazole with respect to thyroid function (26), all patients’ serum thyroid-stimulating hormone (TSH) levels were examined before and after the treatment. Patients were randomly allocated (using a computer-generated sequence) in a double-blind manner to the following 2 groups: those treated with 5% methimazole cream and those with 2% hydroquinone cream nightly for 8 weeks. The topical 5% methimazole cream was prepared in the department of pharmacology at the Iran University of Medical Sciences. The Iran hormone company provided the methimazole powder. The base comprised of cetostearyl alcohol (0.5%), glycerin (5%), soft petroleum jelly (25%), propyl paraben (0.02%), and methyl paraben (0.018%) in liquid paraffin qs 100%. Topical 2% hydroquinone cream was prepared as a water–oil emulsion with a Eucerin base. The hydroquinone powder was prepared by the Sobhan Daru Company. The prepared creams were encoded by a pharmacologist and were placed in identical packages. Neither the dermatologists nor the patients were aware of the package contents. At the end of study, to analyze the data, the codes of the different creams (concealed in sequentially numbered envelopes) were obtained from the pharmacologist.
All patients were advised to apply sun block every 2 to 3 hours, during daytime.
To calculate MASI scores, the face was divided to 4 regions: forehead (F) 30%, right malar (MR) 30%, left malar (ML) 30%, and chin (C) 10%. Each region was given a numerical value (A, 0 to 6). The severity of the darkness of melasma (D, 0 to 4) and its homogeneity (H, 0 to 4) were summed and multiplied by the numerical value and percentage for each region. Finally, these values were added to obtain the MASI score by a single-blind dermatologist. The full equation was as follows:

This research used the VisioFace software to calculate ΔE scores from VisioFace photography. The ΔE score is a parameter that classifies the sensed color difference; the final ΔE score is the average difference of 8 points (2 points on the forehead, 4 on the cheeks, and 2 on the chin) from a comparison point on the mid glabella in the sagittal plane.
Patients were assessed on the basis of the following lightening categories: mild (< 25%), moderate (25% to 50%), good (50% to 75%), and excellent (> 75%). A reduction in MASI and ΔE scores was valuable to patients and was considered to be a response to treatment.
All adverse effects were listed by the investigators throughout the study.
2.3. Statistical Analysis
Results are presented as mean ± standard deviation (SD) for quantitative variables and are summarized by absolute frequencies and percentages for categorical variables. Categorical variables were compared using chi-square test or Fisher’s exact test. The normality of the data was analyzed using the Kolmogorov-Smirnov test. Quantitative variables were also compared using t-test or Mann-Whitney U test. Multivariate logistic regression analyses were undertaken to examine the association between treatment and outcome in the presence of confounding factors. For statistical analysis, the SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL) was used. P values of ≤ 0.05 were considered statistically significant.
3. Results
Of 58 patients included in this study, 55 completed the study. One patient left the study owing to pregnancy and 2 others were excluded due to loss of availability. The average age of the patients in the methimazole and hydroquinone groups was 31.04 ± 6.83 and 33.07 ± 9.89, respectively. The data showed no statistical differences between the 2 groups (P = 0.376). Table 1 shows the baseline demographic characteristics of each group.
| Variable | Value |
|---|---|
| Age | |
| Methimazole group | 31.04 ± 6.83 |
| Hydroquinone group | 33.07 ± 9.89 |
| Gender | |
| Male | 0 |
| Female | 55 |
| Duration of Melasma (years) | |
| Methimazole group | 7.12 ± 4.06 |
| Hydroquinone group | 8.86 ± 3.84 |
| Family history | |
| Methimazole group | 20 (71%) |
| Hydroquinone group | 22 (81%) |
The subjective assessments (patient satisfaction) of methimazole versus hydroquinone groups (Figure 1) were as follows: excellent, 28.4% versus 22.2%, good, 39.3% versus 48.1%, moderate, 17.9% versus 18.5%, and mild, 14.3% versus 11.1%. The data showed no statistical differences between the 2 groups (P = 0.942).
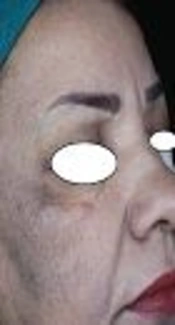
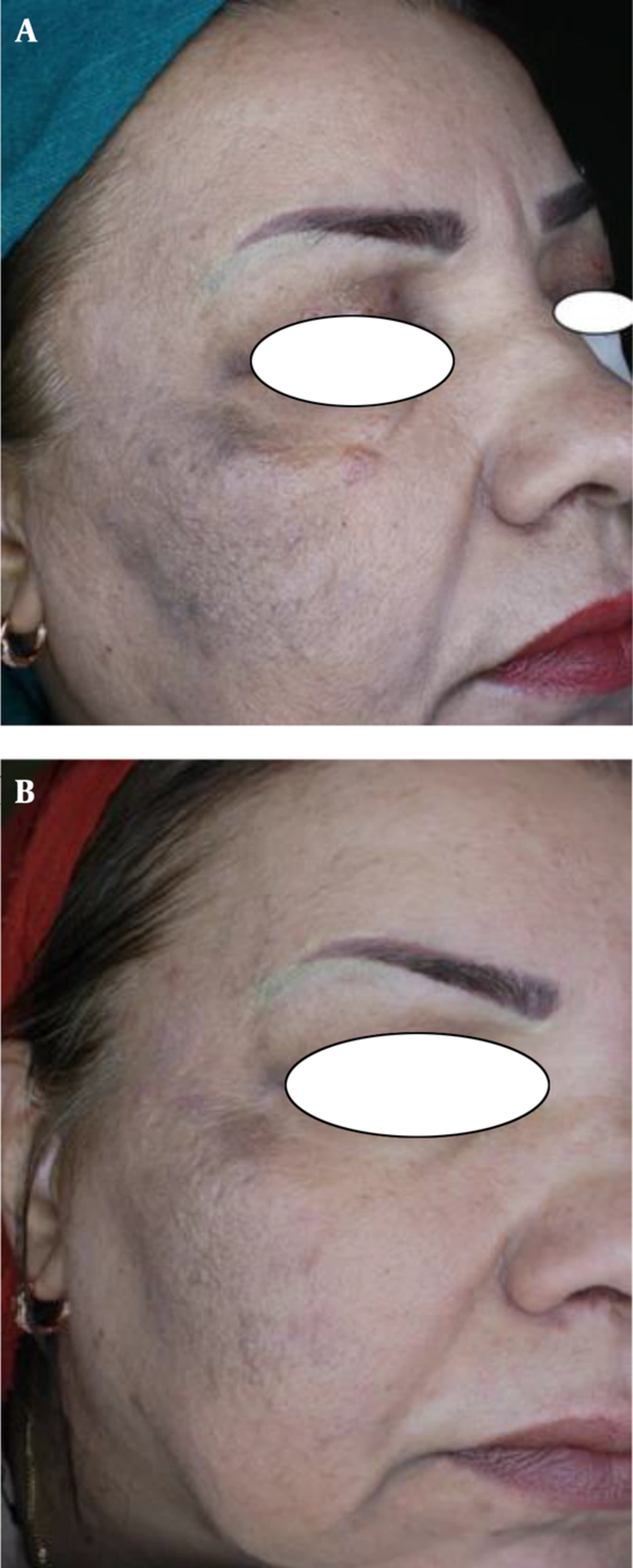
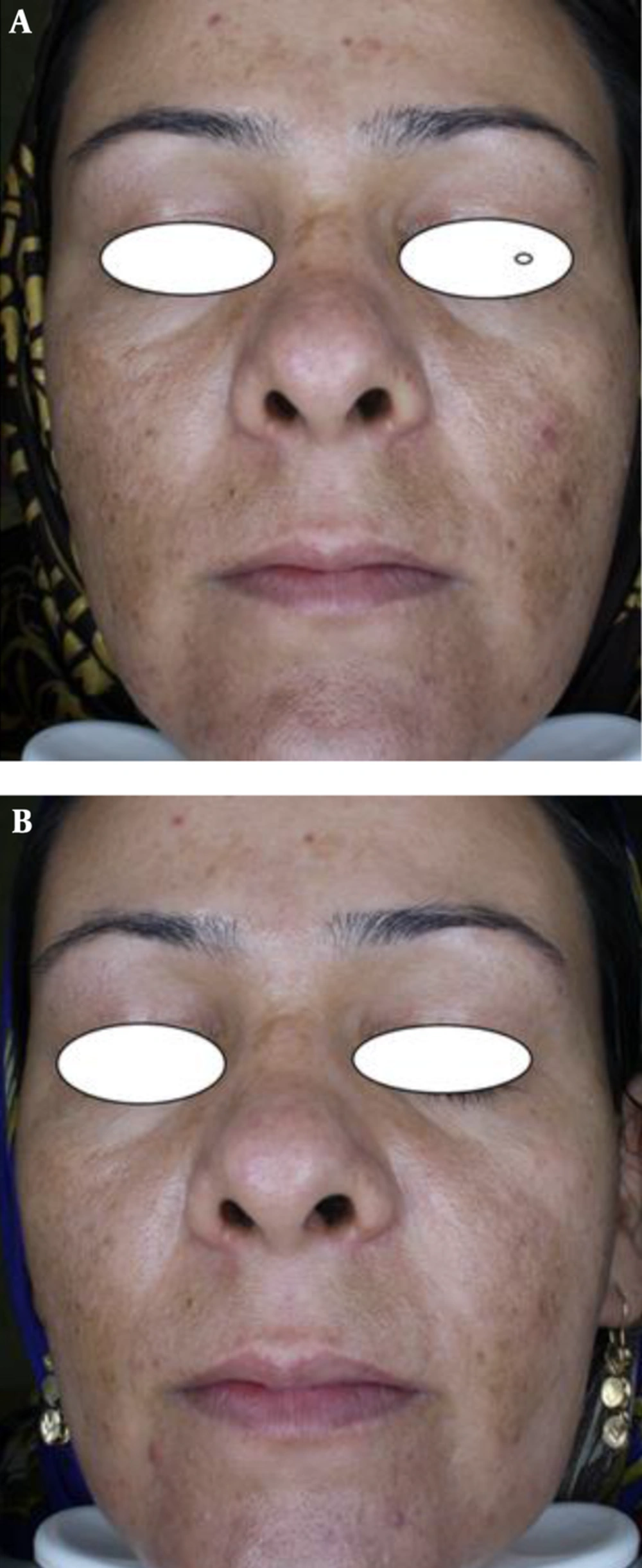
The first objective response to treatment was the reduction in MASI scores after 8 weeks. Before treatment, the average MASI scores for the methimazole and hydroquinone groups were 12.36 ± 5.37 and 15.38 ± 6.19, respectively, showing no statistical differences between the 2 groups (P = 0.069). After the treatment, the average MASI scores were 6.46 ± 3.98 and 8.88 ± 5.38, respectively, showing significant statistical differences between the 2 groups (P = 0.042).
The second objective response to treatment studied was the decrease in VisiFace ΔE score after 8 weeks of treatment. Before the treatment, the average ΔE scores in the methimazole and hydroquinone groups were 8.54 ± 5.14 and 10.80 ± 6.82, respectively, showing no statistical differences between the 2 groups (P = 0.173). After the treatment, the average ΔE scores were 4.41 ± 3.03 and 6.52 ± 5.20, respectively, showing significant statistical differences between the 2 groups (P = 0.049).
The safety of the treatment was assessed by considering serum TSH levels. The initial levels in the methimazole and hydroquinone groups were 3.71 ± 4.17 and 2.84 ± 1.28, respectively, showing no statistical differences between the 2 groups (P = 0.30). After the treatment, average TSH levels were 3.04 ± 1.69 and 2.84 ± 1.28, respectively, showing no statistical differences between the 2 groups (P = 0.613). In the hydroquinone group, both the mean and SD of TSH levels was the same before and after the treatment.
A comparison of the objective responses to treatment and TSH levels between the 2 groups is shown in Table 2.
| M Group Before Treatment | M Group After Treatment | H Group Before Treatment | H Group Before Treatment | P Value Within M Group | |
|---|---|---|---|---|---|
| MASI | 12.36 ± 5.37 | 6.46 ± 3.98 | 15.38 ± 6.19 | 8.88 ± 5.38 | 0.0001 |
| ΔE Score of visioface | 8.54 ± 5.15 | 4.41 ± 3.03 | 10.80 ± 6.8 | 6.52 ± 5.20 | 0.0001 |
| TSH | 3.71 ± 4.17 | 3.04 ± 1.64 | 2.84 ± 1.28 | 2.84 ± 1.28 | 0.351 |
Throughout the treatment, no adverse effects were reported by either group.
4. Discussion
As previously discussed, methimazole has been recently suggested as an effective and efficient drug for reducing pigmentation in humans. Methimazole (1-methyl-2-mercaptoimidazole) is an anti-thyroid agent that is orally administered for treating hyperthyroidism. When used topically, methimazole inhibits melanin production and produces skin depigmentation in both laboratory animals (16) and human subjects (17). Melanocyte cell culture studies have shown that methimazole exerts its depigmenting effect by inhibiting melanin synthesis and not by a melanocytotoxic effect (18). Most probably, both in thyroid cells and melanocytes, methimazole accomplishes metabolization by inhibiting the peroxidase present in cutaneous melanocytes and inhibiting the metabolization of several melanin intermediates, such as dihydroxyphenylalanine (DOPA), dihydroxyindole, and benzothiazine. Thus, the inhibition of peroxidase by methimazole could interfere with different steps in the biosynthesis of eumelanin and pheomelanin pigments (22).
Methimazole is also an inhibitor of mushroom tyrosinase (23, 24), and thus, could theoretically block the hydroxylation of tyrosine to DOPA in melanocytes, the key step in melanin biosynthesis. In contrast, there are concerns about its adverse impact on thyroid glands via systemic absorption.
Until now, there has not been a clinical trial to investigate the effect of methimazole on patients with melasma. Therefore, this study was designed to compare the effectiveness and safety of methimazole versus a conventional therapy (2% hydroquinone). The results showed that methimazole was more effective than hydroquinone and had no effect on TSH levels, demonstrating the effectiveness and safety of methimazole in a human clinical trial. With these results, methimazole can be considered as the first-line therapy for melasma.
Unfortunately, there have been no similar studies on human subjects. Only a few studies about the effectiveness of methimazole in reducing pigmentation have been published. Malek et al. (15) reported in 2013 that 2 patients with hydroquinone-resistant melasma had responded to topical methimazole after 8 weeks. In that study, themethimazole cream base was different from the current study, yet in both studies, the methimazole base had no lightening characteristics. Malek et al.’s study was the only case report in this regard and does not compare the effects of methimazole for melasma with other drugs, yet its treatment protocol was similar to that of the current study. The current study used one subjective and 2 objective assessments (patient satisfaction, MASI score, and ΔE VisiFace score) for data analysis; hence, the results are stronger than those of Malek et al.’s study. In 2008, Kasraee et al. (26) showed that long-term use of methimazole had no significant effect on serum thyroid hormone levels, as confirmed by the current study.