1. Context
Recently, skin cancers have become common in white populations. Melanoma is one of the prevalent and aggressive types of skin cancer, which is more common among the youth. Small noncoding regulatory RNA molecules (miRNAs) have been documented as primary biomolecules in cancer therapy, especially melanoma treatment. MiRNAs are responsible for several molecular modifications in abnormal cells. Involvement of miRNAs in melanoma has been approved by many researchers (1-4).
Some deregulated miRNAs are responsible for the progression and metastasis of melanoma. Although melanoma has been identified in recent years, there are no accurate treatments for this prevalent cancer. Evaluation of miRNAs in various studies in recent decades has shown their precise role in prognostic, diagnostic, and therapeutic processes. In this review, we aimed to evaluate the relationship between miRNAs and related targets involved in melanoma in order to elucidate the molecular mechanisms of this type of skin cancer.
2. Evidence Acquisition
In the past decades, prevalence of skin cancers, particularly melanoma, has grown worldwide. The incidence rate of melanoma has increased over the past 30 years, and the youth seem to be more vulnerable to this type of cancer. It is noteworthy that white populations are more at risk of this aggressive skin cancer (5, 6). Generally, this aggressive type of skin cancer has been identified for many years. However, there are no therapies for the malignant stages of this cancer. Therefore, novel procedures are required for the development of diagnostic and prognostic strategies (7, 8).
According to recent studies, molecular changes lead to modifications of normal cells into abnormal ones. In recent research, miRNAs have been implicated as liable biomarkers of molecular modifications in cancer. MiRNAs are short, noncoding RNAs containing 18 - 22 nucleotides, which bind to the 3’UTR region of target mRNA to prevent its translation into proteins. These new biological molecules can act as negative regulatory factors of gene expression by degrading targeted messenger RNA (mRNA) or inhibiting mRNA translation.
Recently, many studies have been performed on the role of miRNAs in the diagnosis, prognosis, and treatment of cancers, especially melanoma. Furthermore, these small biomolecules are essential for gene regulation in different types of cancers. MiRNA clusters, pre-miRNAs, and mature miRNAs have been examined in recent research and have been shown to regulate some functions in melanoma. In this review, we aimed to briefly elucidate the importance of these remarkable diagnostic, prognostic, and therapeutic biomarkers in melanoma (9-13).
2.1. MiRNA Upregulation in Melanoma
2.1.1. Cell-Cycle Regulatory Overexpressed miRNAs
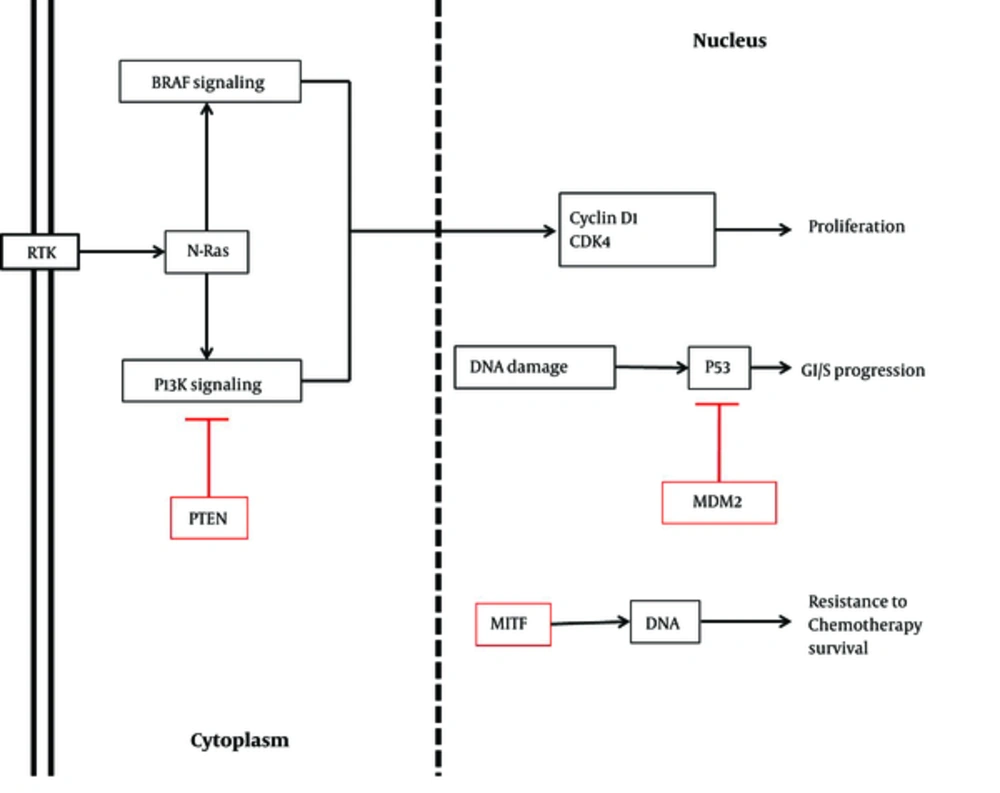
There are several genes and mRNAs regulating cell proliferation, which can be modified by miRNAs. MiR-21, one of these regulatory factors, shows diverse expression patterns in melanoma cell lines at various stages. However, miR-182 has been shown to be methylated in all stages of melanoma. While miR-21 is upregulated in primary melanomas, its expression is prevented in other stages. Furthermore, this miRNA identifies phosphatase and tensin homolog (PTEN), Akt, Bax, and Bcl-2 proteins and induces cell proliferation through different processes, such as phosphorylation, inhibition, and overexpression (Figure 1).
Similar to miR-21, miR-195 is expressed differently in various stages of melanoma. For instance, it is downregulated in primary stages, whereas it is overexpressed in progressive stages to facilitate proliferation of melanoma cell lines. The small cell protein, WEE1 G2 checkpoint kinase (WEE1), as a mitotic inhibitor kinase, is another target of the cell cycle inhibitor, miR-195. As demonstrated by Watanabe et al. in 2013, depletion of WEE1 due to miR-195 attachment enhances cell proliferation (1, 14, 15). Furthermore, in a study by Felicetti et al. and Igoucheva et al., miR-221 and miR-222 were shown to be overexpressed. These upregulated miRNAs targeted P27, which is a cell cycle regulator adhering to cyclin D1. This regulator is downregulated in melanoma owing to increased miRNA expression. Tyrosine-protein kinase kit (c-kit), PTEN, and tissue inhibitor of metalloproteinase (TIMP) are other targets of miR-221/222 cluster (Figure 1). The level of c-kit is reduced due to miR-221 blocking process. In normal cells, c-kit modulates microphthalmia-associated transcription factor (MITF) and tyrosinase, which are mutated in melanoma cells. Depending on the cell stage of melanoma, it seems that miR-221 and miR-222 can have various targets (Table 1) (16-20).
| MiRNA | Validated Target | Reference |
|---|---|---|
| Diverse | ||
| miR-21 | BRAF | (1, 21, 22) |
| miR-195 | WEE1 | (1, 21, 22) |
| Upregulated | ||
| miR-221/222 | P27, C-KIT, TIMP, PTEN, BRAF | (16-20) |
| Pre-miR-17-92 | BIM | (1, 23, 24) |
| miR-506-514 | _ | (1, 23, 24) |
| miR-126 | _ | (1, 21, 22) |
| miR-155 | _ | (1, 21, 22) |
| miR-214 | _ | (1, 21, 22) |
| miR-786-3p | _ | (1, 21, 22) |
| miR-210 | HOXA1, TP53I11, PTPN1 | (1, 21, 22) |
| miR-30/30b | _ | (1, 21, 22) |
| miR-25 | PTEN | (25) |
| miR-181 | PTEN | (25, 26) |
| miR-200b | PTEN | (25) |
| miR-92b | PTEN | (25) |
| miR-199a-5p | ApoE | (27) |
| miR-1908 | ApoE | (27) |
| Downregulated | ||
| miR-182 | MITF, FOXO3, CASP2, PTEN, Akt, Bax, Bcl-2 | (1) |
| miR-9 | E-cadherin | (28) |
| miR-let7-b | CDK, BSG, MMP | (1) |
| miR-193b | CDK | (1) |
| miR-205 | E2F1, E2F5 | (20) |
| miR-145 | C-myc, Erbb3 | (1) |
| miR-18b | MDM2 | (16) |
| miR-211 | MITF | (1, 29, 30) |
| miR-196a | HOX-B7 | (1) |
| miR-199a-3p | C-MET receptor | (23) |
| miR-137 | YB1, MITF, c-MET | (23) |
| miR-101 | BRAF | (31) |
| miR-191 | BRAF | (31) |
| miR-338-3p | BRAF | (31) |
| miR-216b | FOXM1 | (32) |
| miR-219-5p | Bcl-2 | (33) |
| miR-625 | SOX-2 | (34) |
The miRNAs and Related Targets Involved in Melanoma
There is some evidence on pre-miRNAs, triggering the alteration of mRNA expression. Upregulation of pre-miR-17 - 92 cluster, as reported by Levy et al., is an explicit example. This cluster is essential for inhibiting the proapoptotic moderator, Bcl-2L11 (BIM). Similar to this cluster, Georgantas et al. reported that miR-506-514 cluster on chromosome X is overexpressed in melanoma (1, 23, 24). Overall, several miRNAs have been reported as key regulators of cell production, including miR-786-3p, miR-214, miR-155, and miR-126 (1).
2.1.2. Immunosuppressive miRNAs
Several miRNAs have been introduced as mediators of immune responses in melanoma. MiR-210, miR-30, and miR-30b suppress immune responses through different processes. Upregulation of miR-210 targets immunosuppressive genes, namely Homeobox A1 (HOXA1), tumor protein P53 inducible protein 11 (TP53I11), and protein tyrosine phosphatase, non-receptor type 1 (PTPN1). Therefore, tumors remain undefined and cytotoxic T lymphocytes cannot destroy them (Table 1).
MiR-30 and miR-30b are overexpressed in melanoma, resulting in an increase in the expression of interleukin-10 (IL-10) and providing an immunosuppressive environment in combination with polypeptide N-acetylgalactosaminyltransferase 7 (GALNT7), as a glycosylated protein. This gene modifies the cell surface of melanoma cells and affects cellular adherence; metastasis occurs due to discontinuity of cancerous cells. Therefore, miR-30b and miR-30 are accountable for metastasis in melanoma (1, 21, 22).
MiR-34a/c is another miRNA, which modulates immune responses in melanoma. These miRNAs downregulate UL16 binding protein 2 (ULBP2), a ligand for natural killer cells (NKCs) in the immune system. According to previous investigations, excess levels of miRNAs in melanoma remove ULBP2, and cancerous cells evade NKCs (1, 35).
2.2. Downregulated miRNAs in Melanoma
While there are overexpressed miRNAs in melanoma, reports have documented approximately 57 downregulated miRNAs. The mounting expression levels of target genes, owing to the reduction of miRNAs, trigger cellular mechanisms, including cell cycle, cell proliferation, and cell adhesion. Downregulation of miR-29c has been reported in metastatic melanoma (36); this study documented some of these miRNAs.
2.2.1. Cell-Cycle Regulatory Downregulated miRNAs
Adjustment of cell cycle is a key factor in arresting abnormal cell division. There are different molecules, which regulate cell cycle, and there are functional noncoding RNAs, which modulate these regulatory cell cycle factors. Different groups of miRNAs, including miR-let7-b and miR-193b, are examples of this short functional molecule. Loss of these miRNAs increases the expression and protein level of cyclin-dependent kinase D1 (CDK), which has been documented in the progression of melanoma (Table 1). In addition, melanoma metastasis decreases by miR-let-7b, which attenuates its essential target, basigin (BSG), and extracellular matrix metalloproteinase (MMP) (1, 37). Similarly, miR-206 reduces the proliferation and invasion of melanoma cell lines by targeting CDK4, cyclin D1, and cyclin C, which are downregulated in melanoma (Figure 1) (38-40).
MiR-205 is generally mutated because of its site (chromosome 1q), which is a hot spot in melanoma. Similar to other miRNAs, this biomolecule targets some cell proliferation mediators, ie, E2F transcription factors. E2F transcription factors are important cell-cycle regulators, upregulated in melanoma. While miR-205 is reduced, E2F1 and E2F5, as targets of miR-205, are overexpressed, thereby increasing cell proliferation.
It has been reported that injection of miR-205, as an in vivo or in vitro therapeutic marker, affects AKT and blocks the proliferation of melanoma cells (28, 41-43). On the other hand, inoculation of miR-205 increases the level of this essential biomolecule, which in turn decreases phosphorylated protein kinase B (PKB) sources by blocking melanoma cell proliferation. Considerably, these miRNAs perform their roles by reducing eukaryotic translation initiation factor (eIF4E) in melanoma cell lines (Table 1). MiR-145 is another moderator of cell cycle with different functions (1, 20, 43, 44). MiR-145 inhibits cell reproduction owing to the adherence of c-myc and Erbb3; this function is prevented due to miR-145 depletion (38-40).
Surface proteins play a key role in cell adhesion, inhibiting cell migration in cancer. E-cadherin is a vital surface protein, which is regulated by many types of miRNAs. MiR-9 is an essential miRNA, downregulated in melanoma. Lack of miR-9 results in the overexpression of NFkB and Snail. Eventually, E-cadherin is inhibited by the mentioned genes (28).
2.2.2. Epigenetic Regulatory miRNAs
Methylation is a regulatory factor for gene expression. Some miRNAs are downregulated, causing methylation. Based on a survey by Mazar et al., miR-375 is used in melanoma cell lines for methylation of demethylated CpG islands with some specific markers. In their study, different stages of melanoma were analyzed, and it was shown that miR-375 is methylated in stages II and III. Similar to miR-375, miR-34b is stage-dependently methylated, while miR-34b is methylated in stages III and IV (45).
MiR-31 is another miRNA, which is methylated and reduced in melanoma. This methylation could be due to the enhancement of zeste homolog 2 (EZH2)-mediated histone methylation or DNA methylation, which can silence genome expression. Chinnaiyan et al. reported reduced levels of genes, namely MET and SRC, which are targeted by miR-31, and recognized them as oncogenes in melanoma cell lines. Therefore, methylation of this miRNA induces melanoma cell proliferation (46).
2.3. Tumor Suppressor miRNAs
There are some tumor suppressor miRNAs, which inhibit oncogenes to prevent tumor metabolism. MiR-18b is one of these regulatory miRNAs, which moderates p53 expression. P53 is a key tumor suppressor in different types of cancers. Mouse double minute 2 homolog (MDM2), a p53 inhibitor, is reduced by miR-18b. The overexpression of p53 occurs through this attachment subsequent to apoptotic-cascade activation (Figure 1). As a result of miR-18b downregulation, p53 is blocked by MDM2 and apoptosis is inhibited (17, 19, 20, 43).
MiR-211 is another tumor suppressor in melanoma, which targets MITF, a significant gene in melanoma, and is downregulated in cancer (Figure 1) (1, 29, 30). Likewise, miR-196a suppresses the development of melanoma. Several targets have been introduced for miR-196a, such as HOX-B7, which is inversely regulated. Overexpression of this gene triggers the basic fibroblast growth factor (bFGF) signaling pathway. Finally, this signaling pathway increases the expression of ETS-1 transcription factor and bone morphogenetic protein-4 (BMP-4), inducing the progression of melanoma (1).
Other examples of tumor suppressor miRNAs include miR-34b, miR-34c, and miR-199a-3p. C-MET receptor is the target factor, which is overexpressed in many aggressive cancers (including melanoma) as a result of miRNA inhibition, leading to invasion and apoptosis inhibition. Another miRNA, known as miR-137, does not only target c-MET, but also targets MITF and Y-box binding protein 1 (YB1), which are modulatory members of cell reproduction and decrease in melanoma. Similarly, reduction of miR-199 and miR-34b/c increases the expression of target genes, c-MET, and consequently, melanoma migration is boosted (Table 1) (19, 23, 24, 47, 48).
Sun et al. introduced another new tumor suppressor, known as miR-216b. It has been reported that miR-216b concretes to Forkhead box M1 (FOXM1). Furthermore, in melanoma cell lines, miR-216b is reduced. Depletion of the mentioned miRNA leads to uncontrolled cell proliferation as a result of FOXM1 excess. Therefore, we could classify this miRNA in the category of tumor-suppressor miRNAs in melanoma (Table 1) (32). Moreover, NRAS, another oncogenic marker in melanoma, is downregulated by attachment of mir-let-7 family. As mentioned earlier, since this miRNA is decreased in melanoma by transfecting to melanoma cancer cells, apoptosis is induced and cell migration is ceased (Figure 1) (4, 49, 50).
2.4. OncomiRs
There are several pathways influenced by genetic mutations. In addition, there are numerous miRNAs requiring these mutations. BRAF mutation is the major mutant gene in most cancers, especially melanoma, which acts through MAPK signaling pathway and is stated to be the major affected pathway in melanoma (3, 4, 51). In a study by Pinto et al., various miRNAs and drugs were investigated to elicit the efficacy of miRNAs in the genetic mutation of melanoma, especially mutations in BRAF. They showed that miR-21, miR-101, miR-191, miR-221, and miR-338-3p bind to wild-type BRAF, while BRAF mutated genes decreased the expression level of these biological noncoding RNAs (Figure 1 and Table 1) (31, 52).
MiR-25, miR-181, miR-200b, and miR-92b are upregulated oncomiRs in melanoma. Similar to other miRNAs, they target particular genes, namely PTEN, a moderator gene of PI3K signaling pathway (25, 26). Multiple miRNAs, including miR-199a-5p and miR-1908, comprise the oncomiR group. Pencheva et al. studied this category and their targets and documented an essential marker, known as apolipoprotein E (ApoE), which is inhibited by the mentioned miRNAs. This protein plays its major role through suppressing metastasis. Due to the combination of ApoE and miRNAs, metastasis and angiogenesis are induced (27).
3. Results
As miRNAs play a key role in the adjustment of melanoma, recent studies have investigated their therapeutic applications. The findings have marked some of these miRNAs as therapeutic biomarkers. In a study by Segura et al. in 2011, miR-182 was investigated in melanoma cell lines of mice. Cells were inoculated by anti-miR-182, which targets miR-182. MITF, FOXO3, and CASP2, as miR-182 targets, increased the levels of gene expression and proteins. Consequently, overexpression of CASP2, a proapoptotic gene in the caspase family, induced melanoma cell apoptosis (Table 1) (1, 53).
Another study on the expression level of miR-219-5p showed that this miRNA could be used as a therapeutic factor in melanoma. MiR-219-5p targets Bcl-2, and its target is overexpressed as it is downregulated in melanoma. However, prevention of melanoma metastasis and cell proliferation is accompanied by miR-219-5p overexpression. Long et al. suggested this miRNA as an option for melanoma treatment (33).
In a study by Li et al. miR-625 was investigated given its role in various cancers, including prostate, breast, and gastric cancers. Considering some assumptions about miR-625 function in melanoma, Li et al. examined its accurate role in melanoma. They reported miR-625 downregulation in melanoma cancerous cells, which caused metastasis. Therefore, it was hypothesized that this miRNA could be used as a diagnostic and therapeutic biomolecule.
Likewise, another miRNA, miR-625, has its own target, namely sex-determining region Y-box 2 (SOX-2). SOX-2 stimulates cell proliferation and invasion when miR-625 is decreased. These findings, along with other prior investigations, represent the possibility of therapeutic application of miR-625 for melanoma (Table 1) (34).
Since drugs used for melanoma treatment are not sufficiently efficacious, more studies have been performed to determine novel methods of therapy. Therefore, importance of miRNAs in the efficacy of drugs (which develop drug-resistant and drug-sensitive cells) was underlined for treatment purposes. Among these miRNAs, miR-125a was introduced as a major biomarker in previous research.
Furthermore, Koetz-Ploch et al. showed that miR-125a was upregulated in melanoma and subsequently prevented apoptotic pathways by inhibiting proapoptotic elements. In addition, overexpression of miR-125a affected other signaling pathways, including TGF-β signaling pathway. They suggested this miRNA as a treatment component in melanoma cell lines, which are resistant to drugs (54).
4. Conclusions
MiRNAs are preferable in the diagnosis of primary and aggressive stages of melanoma. Although previous research has focused on these biomolecules and their efficacy in diagnosis, several investigations have aimed to introduce treatment methods for melanoma considering its rising incidence. In addition to their application in drugs, as mentioned earlier, several studies have documented multiple therapeutic miRNAs, including miR-625, miR-219-5p, and anti-miR-182 (1, 33, 34). Moreover, some diagnostic miRNAs can be used in therapy. For instance, in previous studies, miR-155, which is downregulated in melanoma, was injected in melanoma cell lines to increase its expression (1, 21, 22). Similar procedures can be executed for miR-193b as a practical therapeutic option (1). Considering the affirmative results of miRNA application in recent surveys and introduction of these small noncoding RNAs as feasible therapeutic markers, these molecular techniques should replace traditional methods according to research in this area.