1. Background
Melanoma is the most aggressive and fatal type of skin cancer with high mortality rate in the world (1). Recent studies suggest that photodynamic therapy (PDT), as an adjuvant therapy, could improve current treatment options in advanced melanoma (2). Photodynamic therapy is based on exciting a photosensitizer (PS) with a light source that could generate cytotoxic reactive oxygen species (ROS) in the cells (3). It is believed that this novel therapeutic modality has no harmful side-effects of chemotherapy and radiotherapy, including multi-drug resistance and effect on normal cells (4). Different types of PSs, such as hypericin, Protophorphyrin IX (PPIX), and benzoporphyrin derivatives, showed promising therapeutic effects in skin cancer and melanoma (5). However, these kinds of PSs have several limitations, including low solubility, instability, and short half-life in the tissue, suggesting a need for finding new potent PS (6). In this line, nano-particles, such as metallic NPs, ceramic NPs, and quantum dots could help improve PDT of cancer (7). In particular, experimental results showed that TiO2 NPs could be an excellent PS in PDT of cancer due to their unique properties, such as strong optical absorption, high chemical stability, and non-toxicity (8, 9). Low concentrations of TiO2 NPs did not show significant toxicity by themselves yet, irradiation of these NPs by ultraviolet (UV) light could lead to generation of ROS and consequently cell death. Due to side effects of UV light, many efforts are in progress to improve photocatalytic activity of TiO2 NPs toward safe lights (10). For instance, doping TiO2 with carbon could enhance its photocatalytic properties toward visible light (11, 12). To find more improved TiO2-based PSs, this study aimed to determining effects of graphene oxide (GO)/TiO2 hybrid (GOT) NPs, as a new PS in melanoma A375 cells (13). The GO NPs are very applicable in nanomedicine due to their higher level of water dispersion and great biocompatibility (14).
It has been recently reported that GOT NPs could be used for PDT therapy of cancer via induction of DNA damage and cell death (15). In this study, the researchers aimed at addressing whether GOT NPs might be considered as a PS for PDT of melanoma. To do this, the researchers selected A375 cancer cells and showed that photodynamic GOT NPs could induce cell death in this type of melanoma cells in a concentration-dependent manner.
2. Methods
2.1. Preparation of Nanoparticles and PDT
The GOT NPs (50/50) were provided by Dr. Khataee from Tabriz University. The NPs were autoclaved for 20 minutes and dispersed in RPMI-1640 followed with 10 minutes of sonication. Fetal bovine serum (FBS) (10%) was used as a stabilizer as reported. For PDT, cells were exposed to 1 and 100 µg/mL concentrations of NPs in the dark for 2 hours and irradiated with a Xenon 55 W lamp and an ultraviolet cutoff filter eliminating UV light for 10 minutes (16).
2.2. Cell culture and Morphological Assessment
Melanoma A375 cancer cell line was obtained from the national cell bank of Iran (Pasteur Institute, Tehran, Iran). The cells were cultured in a humidified incubator at 37°C and in 5% CO2. The RPMI-1640 medium (Biosera, UK) supplemented with 10% FBS, 1% penicillin, and 1% streptomycin was used as a complete medium. Morphological changes were studied by an inverted microscope (Olympus, Japan).
2.3. Cell growth and Viability Assays
Trypan blue exclusion assay was used for studying growth and viability, as reported recently (16). The cells were incubated with different concentrations (1 - 100 µg/mL) of GOT for 24 hours, then the number of cells were counted by a hematocytometer under a light microscope (ZEISS, Germany).
2.4. Cytotoxicity Assay
Cell death and cytotoxicity was studied by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, cells were treated with different concentrations of NPs for 24 hours, then 10 µL MTT (5 mg/ml) solution was added to the cells and incubated at 37°C for 2 to 4 hours. Following dissolving formazan products by DMSO, the absorbance was measured by the enzyme linked immunosorbent assay (ELISA) reader (Thermo / LabSystems Multiskan, USA) at 580 nm (16).
2.5. Statistical Analysis
The expression of data was by mean ± standard deviation (SD) for at least 3 independent determinations in triplicates. Analysis was performed by student’s t-test by Microsoft Excel 2010 (Microsoft, Washington, USA); statistically significant differences were considered at P < 0.05.
3. Results
3.1. Effect of GOT on Growth and Viability of A375 Cells
First, the researchers studied effects of different concentrations of GOT NPs on growth and viability of A375 cells with or without light irradiation. While 1 and 100 µg/mL concentrations of NPs had no significant effects on growth and viability, their irradiation with visible light produced significant anti-cancer effects. For example, upon irradiation, growth of the A375 treated with 1 and 100 µg/mL concentrations of GOT was inhibited by 20% and 50%, respectively, after 24 hours of treatment (Figure 1A). With the same condition, viability of the cells treated with 1 and 100 µg/mL concentrations of photodynamic NPs was inhibited by 10% and 25%, respectively (Figure 1B).
Cells exposed to different concentrations (1 and 100 µg/mL) of GOT with or without visible-light radiation for 24 hours, and growth and viability were studied by trypan blue exclusion test. Data are from 3 independent experiments, all performed in triplicates ± SD. Stars are statistically significant data (P < 0.05).
3.2. Cytotoxic effect of GOT on A375 Cells
In order to confirm whether a decrease in growth and viability is related to cytotoxicity, the researchers examined effects of NPs by the MTT test. In agreement with viability results (Figure 1B), treating A375 cells with 1 and 100 µg/mL photodynamic GOT induced 25% and 40% cytotoxicity that was higher than effects of NP alone (Figure 2).
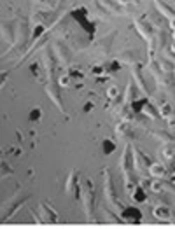
3.3. Morphological Changes of A375 Cells by TiO2/GO Nanoparticles
Effect of GOT on cell morphology changes were also studied (Figure 3). The A375 cells clearly grew with a form of spindle. It was observed that after treating A375 cells with 1 µg/mL concentration of GOT in the presence of light, cell numbers of A375 cells decreased. The rounded cells in irradiated 1 µg/mL GOT may indicate cell death (Figure 3). Amount of cells shrinkage and rounded cells in 100 µg/mL concentration of GOT in the presence of light, was higher than the 1 µg/mL concentration (Figure 3).
4. Discussion
Melanoma is suppressed as one of incur types of skin cancer and any effort for improving available treatment options may be helpful for these patients (2). This study highlights the importance of NP-based PDT, as a new therapeutic strategy that has been recently proposed for different types of cancer (7). In this context, TiO2, owing to the least toxicity, lowest cost, and greatest stability is the most important photocatalytic NP in nanomedicine (17). The PDT application of TiO2 was first reported by Zhang et al., who demonstrated that TiO2 NPs, under UV irradiation, are able to overcome SMMC-7721 hepatocarcinoma cancer cells by producing intra cellular ROS (9). The TiO2 could also have an effect on the treatment of melanoma, as Harada et al. showed that TiO2 NPs, under sonodynamic therapy, could have cytotoxic effects on melanoma C32 mice cancer cells via induction of apoptosis (18). However, TiO2 itself, due to high level of energy gap band, cannot have an effective photocatalytic impact on the presence of visible light (19) and doping TiO2 with another PSs is an effective way to increase photocatalytic effects of TiO2 in the visible region. For example, a recent study showed that by doping nitrogen to titanium dioxide, controllable ROS producing and cell death responses could be observed under the visible light area (16). Current researches indicate that graphene could increase the TiO2 efficiency by trapping the electrons, reducing energy gap bands, and decreasing the recommendation rate of the electron-cavity in TiO2 (20). Furthermore, GO, due to its better water dispersion and its oxygen content, is much better than graphene and other carbon-based NPs (14, 21). Graphene oxide can load different kinds of drug molecules, because of its large number of residual carboxylic acid, epoxide groups, and hydroxide on its surface (19). Reaction between the carboxylic groups of GO and hydroxyl groups of TiO2 forms the basis for the creation of the GOT (14). For these reasons, GO NPs have been proposed for treatment of cancer (5). As an evidence, Fiorillo et al. revealed that GO NPs could eradicate cancer stem cells, offering them as a new therapeutic strategy of cancer (22). Moreover, when GO is co-loaded with a second anti-cancer agent, its anti-cancer properties might be improved (5). The mechanism of GOT action in photodynamic therapy approaches is through an increase in intracellular ROS and ultimately eliciting cell death and apoptosis (19). In line with these results, the current study reports that GOT inhibits growth and evokes cell death merely under visible light irradiation (Figures 1 and 2). These results are similar to the result of Shang et al., who showed that irradiated GO-TiO2 has wider absorption spectrum from UV to visible region and could induce cell death in HepG2 cells more potent than photokilling efficiency compered to TiO2 (15). The researchers also observed that GOT is able to suppress melanoma A375 cancer cells in a dose-dependent manner in such a way that 100 µg/mL had the maximum growth inhibition and cytotoxic effects in melanoma cells (Figure 1). These might be related to different potency of different concentrations of GTO NPs in induction of ROS (16). Nevertheless, effects of visible-light irradiated GOT on growth and death pathways are consistent with effects of GOT in HeLa cells. Mechanistically, it is possible that effects of GTO in the current system might be related to ROS production as recently reported by Zhen Hu et al. (10).
4.1. Conclusion
Taken together, the results show that GO increases TiO2 photocatalytic effects in A375 cancer cells under exposure to visible light in a dose-dependent manner. This significant photodynamic impact supports future surveys of similar graphene-based PSs in photodynamic treatment.