1. Background
Fibroblasts are the most prominent cells of dermis, thus, their senescence and injury can have an influence on the entire skin structure. Since many cosmetic products and approaches claim skin rejuvenation, therefore, examining the effect of some Iranian traditional medicinal herbs and antioxidant ingredients on viability and proliferation of such cells seems important in comparison with new and modern cosmetics approaches for skin rejuvenation and anti-aging effects (1) due to many advantages of herbal ingredients for instance they are natural, available and cheap; they grow up in many climates and can create a lot of job opportunities.
Skin aging is a complicated biological process affected by combination of endogenous and exogenous factors (1), which leads to less cellular viability and proliferation (2, 3).
Anti-age formulas claim skin rejuvenation by their antioxidant capacity, however, the clear parameter is the relationship between senescence, viability, proliferation, and differentiation of cells and ROS. ROS accumulation causes gradual loss of cellular resistance against stresses, damages, and diseases. Aging has been associated with high level of ROS production besides gene expressions and metabolic defects. Enhancement of ROS level has demonstrated the maintenance of cellular senescence process so there is a connection between ROS, aging, and cellular senescence (4).
Consequently, the anti-aging formulas have to be possessed anti-ROS capacity, however, since the role of herbs and also modern anti-age approaches on ROS-level have not been exactly demonstrated yet hence, in this survey, the effect and potential of Rosemarinus officinalis leaf extract, Altheae officinalis root extract and modern anti-aging approaches were assessed due to evaluating the cellular response to these herbs. Basic fibroblast growth factor (bFGF) and L-butionine sulfoximine (L-BSO) have been employed as oxidants to measure the cellular antioxidant capacity.
These factors lead to progressive structural and physiological changes in skin layers as well as changes in skin appearance, particularly on the sun-exposed areas of skin (5).
2. Methods
2.1. Isolation of Human Dermal Fibroblasts and Cell Culture Establishment
Foreskin sample of a circumcised newborn is obtained under sterile condition in PBS and transported to the laboratory on ice. The sample was cultured in Dulbecco’s Modified eagle medium (DMEM) (Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 1% L-glutamine (Sigma, USA), and 10% penicillin/streptomycin (Sigma, USA). The dermis separated under a laminar hood by 0.25% Trypsin-EDTA (1X) (Gibco, USA) in to 1mm pieces by sterile scissors and explanted into a 100-mm cell culture dish (Corning, USA) containing DMEM/10%, FBS/1%, L-glutamine/1%, and penicillin/streptomycin and incubated at 37°C, 5% CO2, and 95% of humidity. The medium was changed whenever it was needed (in case of color change in culture medium). A total of 90% of confluent cells were harvested by tripsinization after keeping them in the incubator for 1 minute.
2.2. Plant Material
The root of Althaea officinalis and leaves of Rosmarinus officinalis were procured from a local market and identified by comparing standard herbarium specimens available in Islamic Azad University, pharmaceutical sciences branch, Tehran, Iran. Voucher numbers: Rosemarinus officinalis L. is 250-PMP/A and Altheae officinalis is 312-PMP/A.
These parts of the plant are crushed in a mixer and passed through the sieve number 80. The various powder drugs were subjected to pharmacognostic studies for confirmation.
The herbs used in the present study for making herbal extracts were pre-washed, dried, crushed, grinded, and passed through 100 mesh stainless steel sieves after 72 hours of maceration in 70°C (Indirect heat) to obtain a homogenous sample and water was used as a base. The extracts were prepared utilizing this methodology; the extracts were filtered through muslin cloth then through filter paper under vacuum. The extracts were used immediately.
2.3. Herbal Extracts’ Standardization
To standardize the herbal extracts thin layer, chromatography was used and performed to verify the exact identity of the components discovered in the qualitative chemical tests. For this aim the chromatography layers were prepared at first. The absorbent powder with volatile solvent, such as acetone, was deposited on a thin layer of a glass plate. After its dryness, a small drop of herbal extract sample was poured at the lowest area of the column and allowed it to separate the various layers. The active ingredients of Rosemarinus officinalis are rosemarinic and caffeic acid and the active ingredient of Altheae officinalis are flavonoids, thus, the samples were compared with these standardized pure materials.
2.4. Measurement the Level of Reactive Oxygen Species (ROS)
ROS-level was assayed by the ROS measurement kit (MGT-M1049, Marker Gene technologies, Eugene, OR, USA). The reagent was the fluorescent substrate, 2 - 7-dichlorofluorosine di-acetate, and buffer solution.
2.5. Graphs Preparation
Graphs are prepared based on the average of living cells in different concentrations of 3 groups: aqueous extract of leaf of Rosemarinus officinalis, aqueous extract of root of Altheae officinalis, the mix of 2 herbs as the 3rd group, and bFGF and LBSO at days 2, 7, and 14. Considering that the Kolmogorov-Smirnov test confirmed the data of different concentrations of 3 groups are normal (P value > 0.05), to compare the different concentrations of various groups, One-way ANOVA and Tukey’s post hoc tests are used.
2.6. Determination of Herbal Extracts Lethal Concentration 50
The herbs used in the present study for making herbal extracts were dried, crushed, and passed through 80 mesh stainless steel sieves; water was used as the base. The extracts were prepared and their LC50 was assessed as described previously (6). Treatments were designed in 6 groups 3 times 5, 10, 20, 30, 50, and 100 µL/mL for Rosmarinus officinalis leaf extract and 5, 15, 25, 50, and 100 µL/mL for Althaea officinalis root extract.
2.7. Preparation of Combined Drug Herbal Extracts Formulation
After selection of method for preparation, multi ingredients of effective concentrations, based on the preliminary physical and biological screening was prepared. The method selected was the direct measurement method and 6 different formulations having concentrations 5, 15, 25, 50, 100, and 200 µL/mL of extracts were prepared for maximum activity (Table 1).
| Herbal Extracts | LC50, µL/mL | Optimum Dosages, µL/mL | |
|---|---|---|---|
| Rosemarinus officinalis leaf extract | 30 | 20 | 10 |
| Althaea officinalis root extract | 50 | 25 | 15 |
| Rosemarinus officinalis leaf extract + Althaea officinalis root extract | 25 | 15 | 5 |
2.8. Brdu Cell Proliferation Assay
Cell proliferation was estimated in HDFs culture at different concentrations (10 and 20 µL/mL of Rosmarinus officinalis leaf extract, 15 and 25 µL/mL of Althaea officinalis, 5 and 15 µL/mL of combination) of herbal extracts using a colorimetric bromodeoxyuridine (Brdu) kit (Roche Diagnostics, East Sussex, UK), according to the previous study. Briefly, cells labeled with 10 µM Brdu for 2 hours at RT were fixed and made permeable with the FixDenat solution for 30 minutes, then incubated with monoclonal anti-Brdu peroxidase-conjugated antibody for 90 minutes. The cells were then washed 3 times with wash buffer and then peroxidase activity was measured using tetramethyl-benzidine as a substrate. The reaction was terminated by adding 25 µL of 1 M H2SO4. Absorbance at 450 nm was recorded in an ELISA plate reader. To study the rate of cell proliferation in the Brdu assay, the number of cells was adjusted to 20,000 to obtain an acceptable optical density (OD) in treatment groups.
3. Results
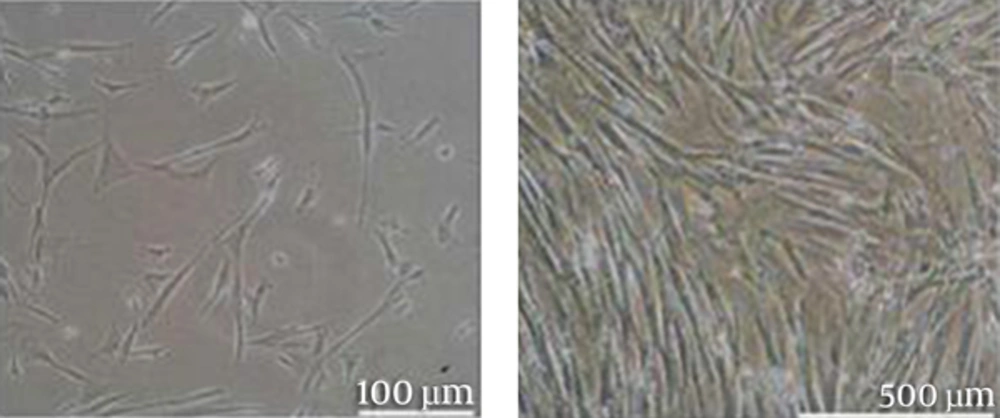
3.1. Isolation and Characterization of HDF
The HDFs migrated out from tissue and adhered to the surface of the culture plate (Figure 1). The fibroblasts at passage 3 were shown in Figure 1.
3.2. Herbal Extracts Induced the Viability of Human Dermal Fibroblasts
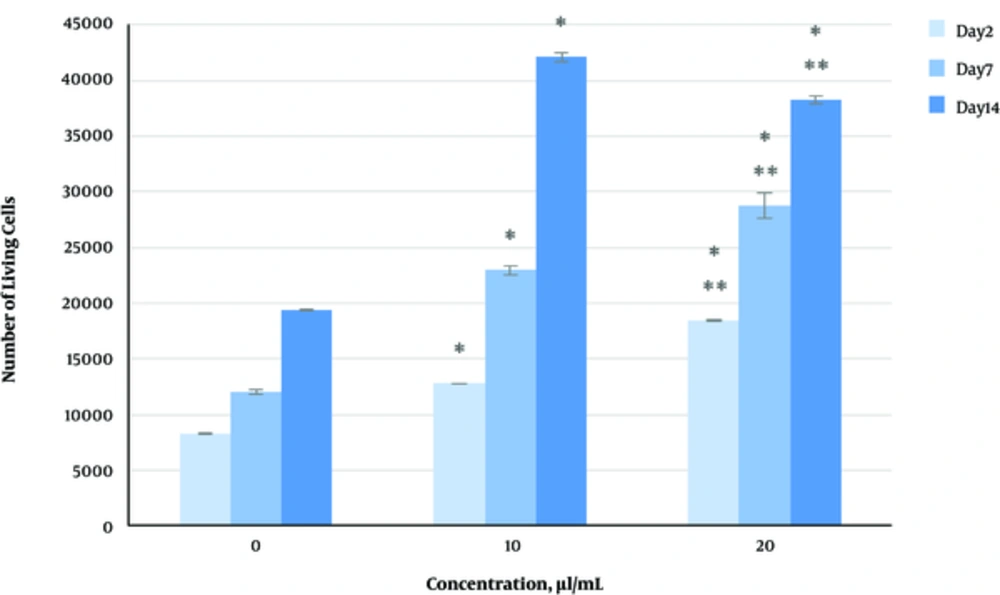
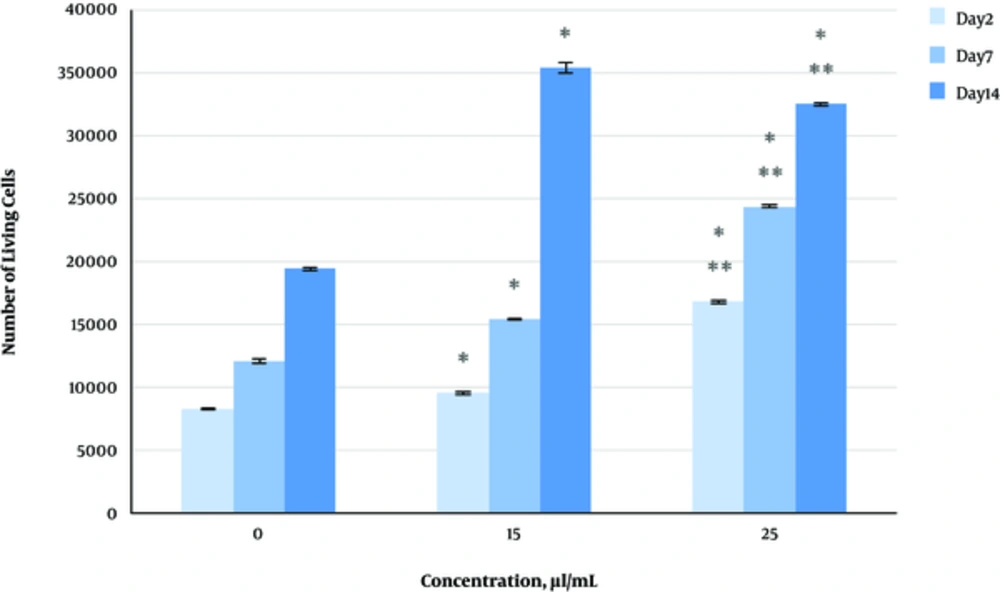
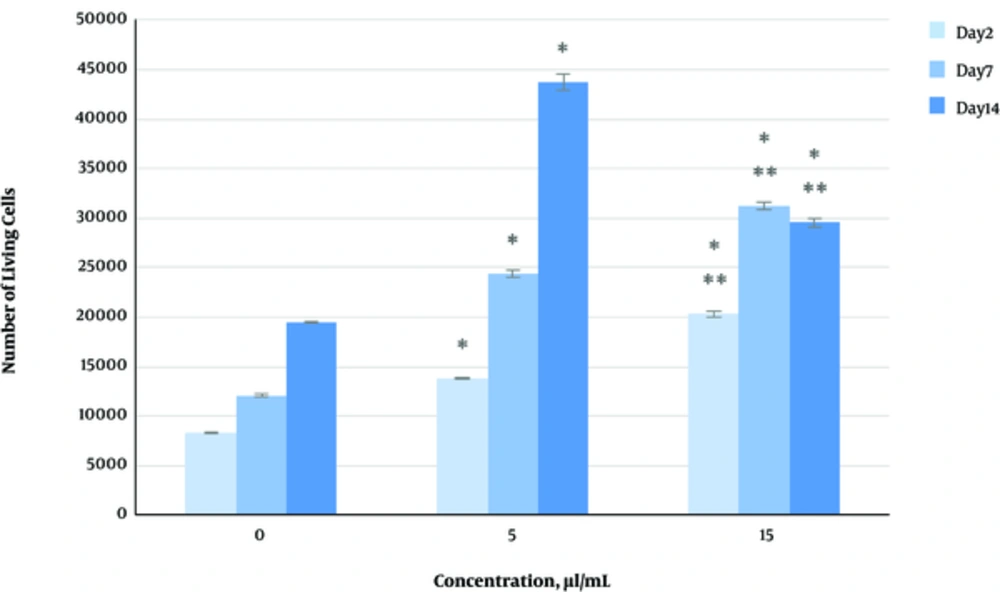
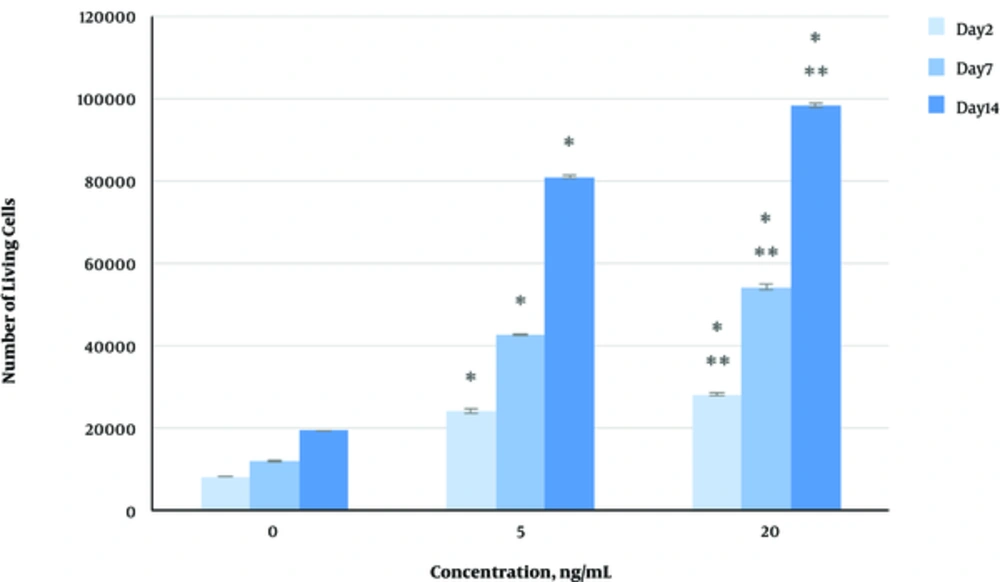
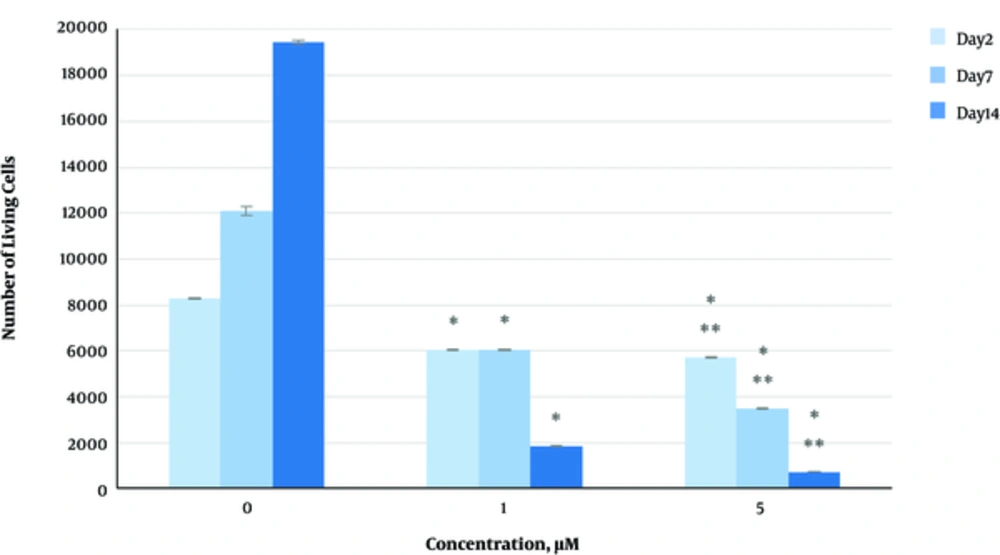
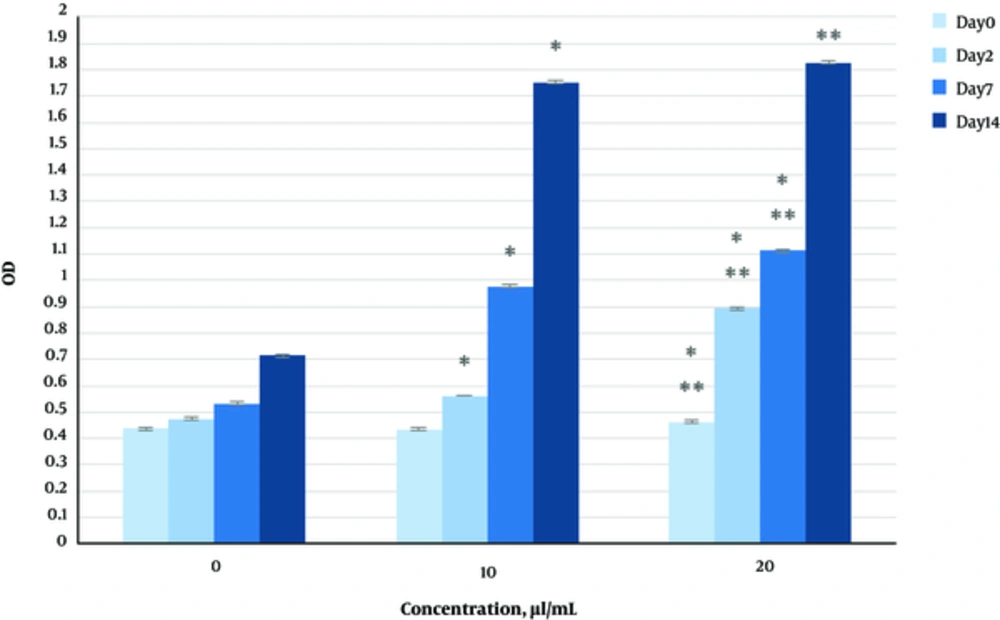
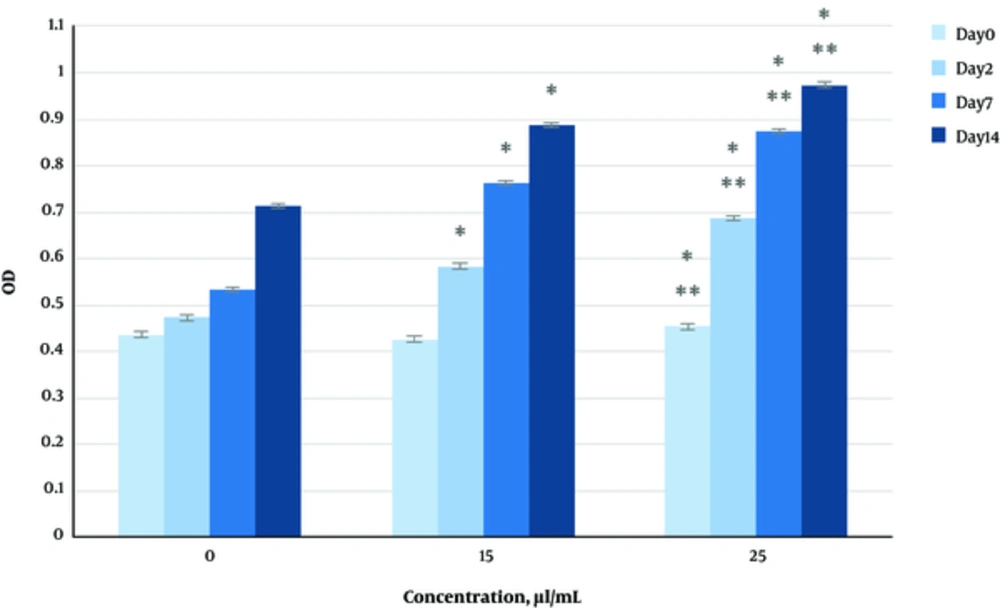
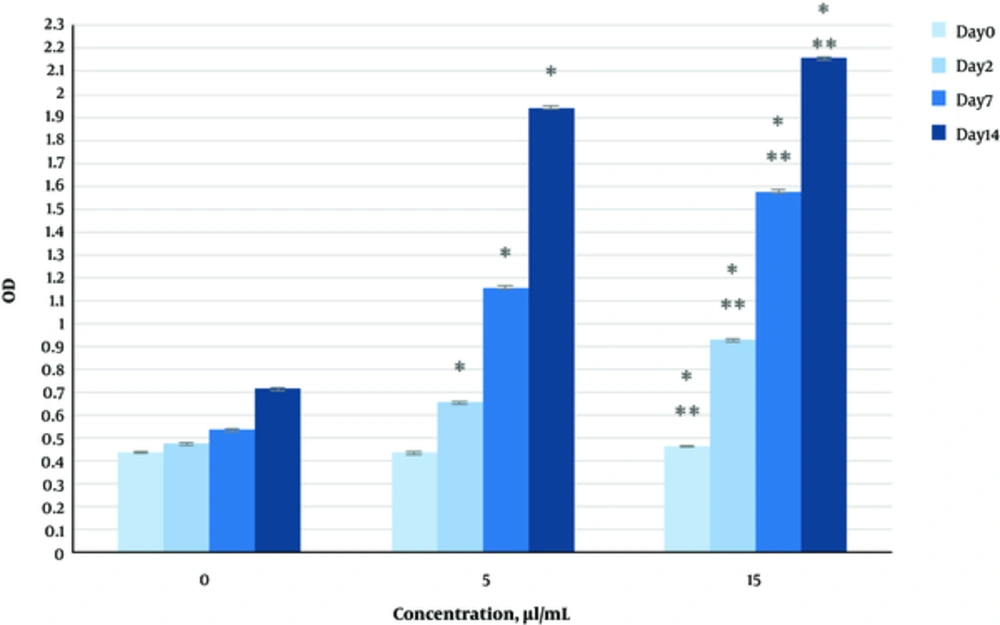
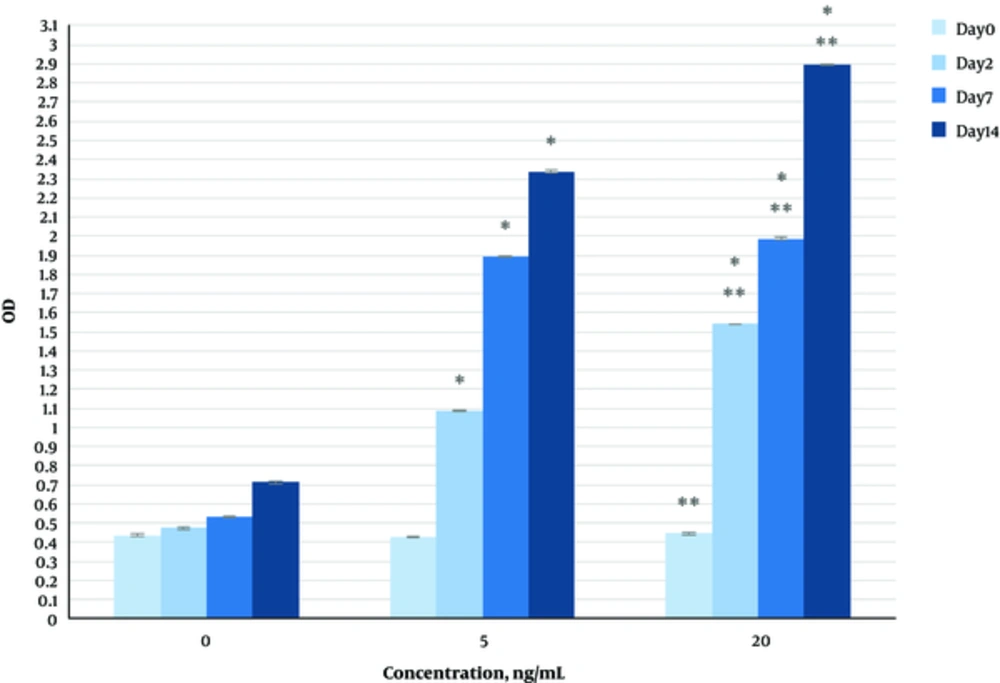
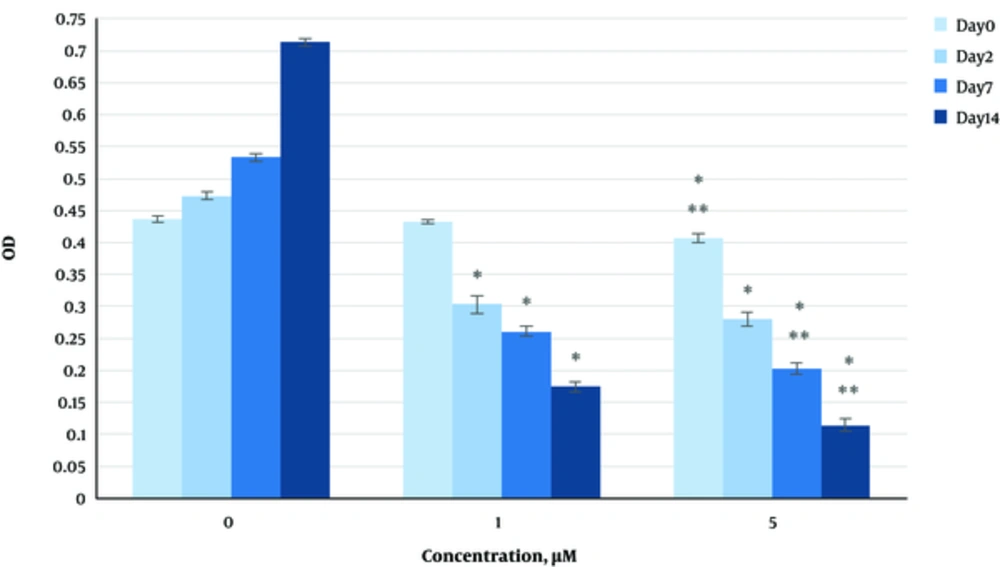
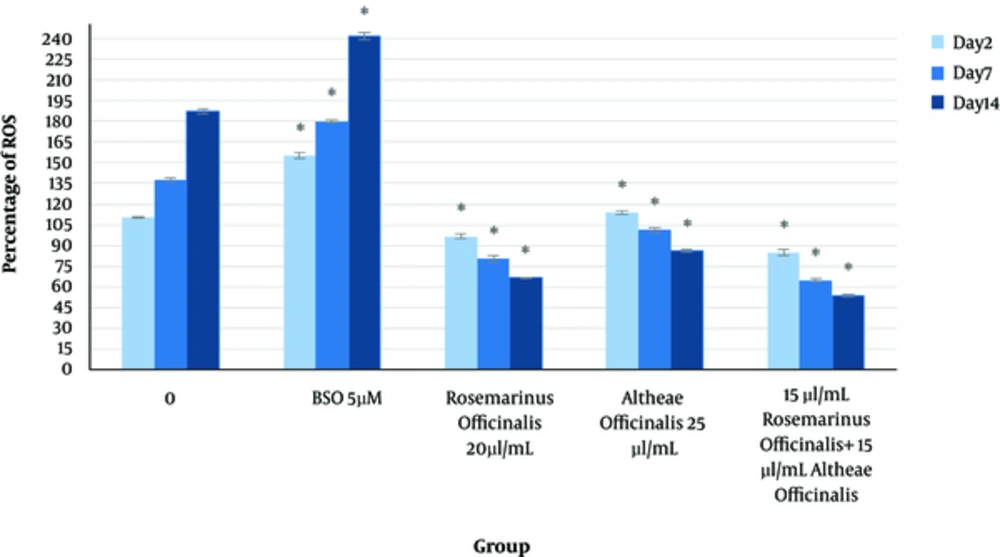
The effect of herbal extracts on the HDFs viability was examined. HDFs were treated by various concentrations of herbal extracts, LBSO and bFGF for 14 days and cell viability was measured by tripan blue method at days 2, 7, and 14. Tripan blue results showed a concentration dependent increase in cellular viability in the HDFs, following exposure to Altheae officinalis extract, however, not Rosmarinus officinalis extract. As it is observed in Figure 2, viability inducing effects of Rosmarinus officinalis leaf extract on HDFs started at 10 µL/mL and increased by 20 µL/mL but then decreased at day 14 in 20 µL/mL (from 1.5, 1. 8 and 2.2% in 10 µL/mL to 2.2, 2.39 and 1.96% in 20 µL/mL at day 2, 7, and 14 vs. control, respectively; P < 0. 01, one way ANOVA). In addition, as observed in Figure 3, viability inducing effects of Althaea officinalis root extract on HDFs started at 15 and increased by 25 µL/mL (from 1.1%, 1.25%, 1.8% in 15 µL/mL to 2%, 2%, 1.6% in 25 µL/mL at day 2, 7, and 14 vs. control, respectively; P < 0. 01, one way ANOVA); eventually, as observed in Figure 4, viability inducing effects of combination of both herbal extracts on HDFs started at 5 and increased by 15 µL/mL (from 1.6%, 2%, 2.3% at 5 µL/mL to 2.5%, 2.58%, 1.55% in 15 µL/mL at day 2, 7, and 14 vs. control respectively; P < 0. 01, one way ANOVA). In Figure 5, as it is observed, viability inducing effects of bFGF on HDFs increased regularly (it started from 3%, 3.5%, and 4.15% in 5 ng/mL to 3.5%, 4.5%, and 5.06% in 20 ng/mL vs. control, respectively; P < 0. 01, one way ANOVA). Inhibitory effect of LBSO started at 1 μM and increased in 5 μM (from -1.36, -1.99 and -10.562 in 1 μM to -1.44, -3.45 and -27.177 in 5 μM) as it is shown in Figure 6.
3.3. Herbal Extracts Induced the Proliferation of Human Dermal Fibroblasts
The effect of herbal extracts on the HDFs proliferation was examined. HDFs were treated by various concentrations of herbal extracts, bFGF and LBSO for 14 days and cell proliferation was measured by BrdU methods at days 0, 2, 4, and 14. BrdU assays showed a concentration dependent increase in cell growth activity in the HDFs, following exposure to Altheae officinalis extract, however, not Rosmarinus officinalis extract. As it is observed in Figure 7, proliferation promoting effects of Rosmarinus officinalis leaf extract on HDFs started at 10 µL/mL and increased by 20 µL/mL (from 0.13, 0.54 and 1.32% in 10 µL/mL to 0.43, 0.65 and 1.36% in 20 µL/mL at days 0, 2, 7, and 14 vs. control respectively; P < 0. 01, one way ANOVA); also, as observed in Figure 8, proliferation promoting effects of Althaea officinalis root extract on HDFs started at 15 and increased by 25 µL/mL (from 0.15, 0.34, 0.46 in 15 µL/mL to 0.23, 0.42, 0.52 in 25 µL/mL at days 0, 2, 7, and 14 vs. control, respectively; P < 0. 01, one way ANOVA); eventually as observed in Figure 9, proliferation promoting effects of combination of both herbal extracts on HDFs started at 5 and increased by 15 µL/mL (3 times more than control at 5 µL/mL to 3.2 times more than control in 15 µL/mL vs. control, respectively; P < 0. 01, one way ANOVA). Figure 10, the rate of HDFs’ proliferation in minimum and maximum dosages of bFGF at days 0, 2, 7, and 14 of treatment was increased and eventually, as it is shown in Figure 11, the rate of HDFs proliferation decreased by LBSO vs. control (P < 0. 01, one way ANOVA) (Figures 12 and 13).
4. Discussion
In this survey we were going to know the power of herbs on cell proliferation and also cell viability in comparison with growth factor and the effectiveness of ROS on cell proliferation and viability considering the important role of ROS on many cellular events. Cell proliferation and also cell viability increased in presence of herbal extracts and bFGF as it is shown in results.
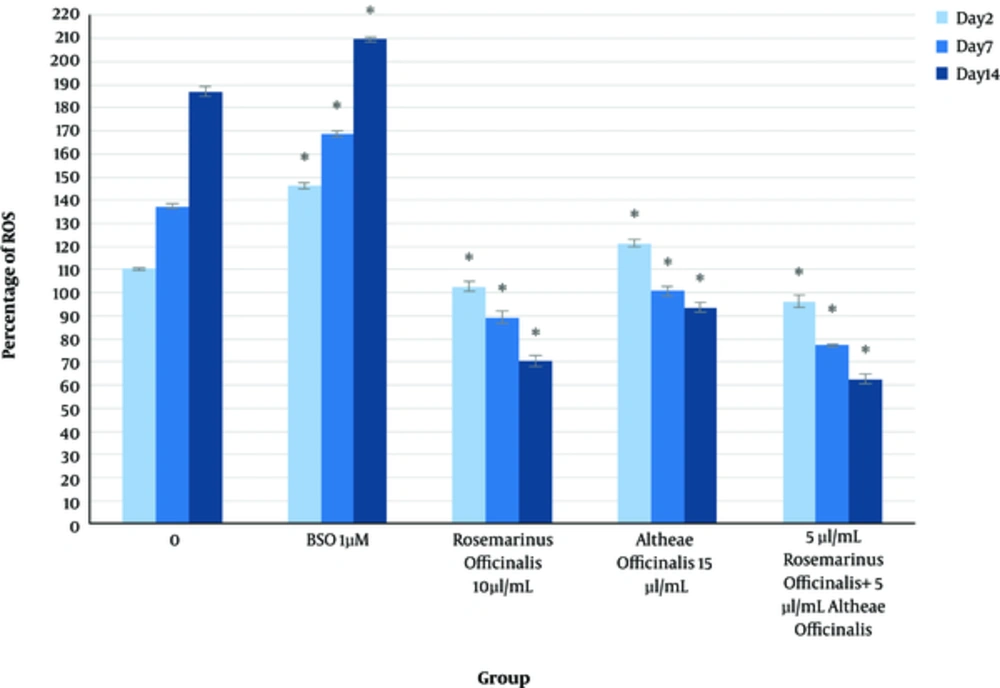
It has been approved that ROS is one of the most important factors in aging. Aggregation of toxins in cells, due to behavioral, environmental, genetics, and internal factors, are susceptible for increasing ROS generation, which leads to aging (4).
A significant increase in intracellular reactive oxygen species (ROS) production, via the PI3K/Akt-Rac1- JNK pathways was observed in dermal fibroblasts, following bFGF treatment (7).
We examined and assessed ROS generation, viability, and proliferation of human dermal fibroblasts in the presence of L-BSO, to confirm that ROS will increase in presence of such oxidant, as it is previously described ROS-level increase in presence of bFGF.
bFGF is a growth factor, which is applied for skin rejuvenating therapies, therefore, the comparison of the effect of this factor, with the effect of herbal extract in ROS generation, seems important due to the fact that ROS increases in proliferation and differentiation. As it is discussed the enhancement of ROS in presence of bFGF has been confirmed, however, it may also have the potential to produce cancerous cells but as it is shown before, the herbal extracts can increase cellular viability and proliferation, however, they decrease the level of ROS generation.
To our knowledge, the proliferation effects of herbal extracts in dermal fibroblasts have been previously elucidated because of the importance of fibroblasts on wound healing (8).
We hypothesized that the skin aging may be due to loss of viability and proliferation capacity of fibroblasts. Based on these lines, this study investigated the effects of herbal extracts for treating skin aging by herbal remedies in comparison with modern anti-aging approaches. In this study, we established an in-vitro fibroblast culture model to clarify the effects of herbal extracts on fibroblast viability and proliferation.
Our results are consistent with several previous studies that have demonstrated the multipotency of rat or human skin dermal fibroblasts (9-13).
In this study, herbal extracts were found to induce significant proliferation and viability of human dermal fibroblasts at concentrations ranging from 5 to 25 µL/mL.
The study by Phan et al. demonstrated the enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin) for treating wounds (8).
Singh et al., been investigated the effect of Terminalia chebula on proliferation of Keratinocytes and Fibroblasts cells and showed that optimized concentration of T. chebula extract on both types of skin cells could be used as a bioactive component for wound healing applications by increasing cell proliferation and decreasing free-radical production without affecting the normal cellular matrix (14).
Negahdari et al. investigated wound healing activity of extracts and formulations of Aloe vera, Henna, Adiantum capillus-veneris, and Myrrh on mouse dermal fibroblast cells and demonstrated that the expression of TGFβ1 gene has been improved. All used extracts upregulated the expression of VEGF-A gene and promoted the migration of mouse fibroblast cells in vitro (15).
Ahmadi Ashtiani et al., evaluated the potential of herbal extracts on dermal papilla cell proliferation of human hair follicle. This survey showed a significant increase in the proliferation of human DPCs at optimum concentrations of a blend of herbal exttract. These results showed that the herbal extracts tested affected the protein expressions of ERK, Akt, cyclin D1, Cdk4, Bcl-2, and Bax in DPCs (16).
The effect of herbal extract on cancerous cell proliferation studied for years and the results on DMBA induced cancerous cells showed that flavonoids of Rosemarinus officinalis and Altheae officinalis are able to activate PPARɣ, which leads to inhibition of COX-2 over expression (that prevents apoptosis and induce cell proliferation). Rosemarinic acid that exist in Rosemarinus officinais extract is able to do it by decreasing COX-2 inhibition (17) in another study, a combination of ethanolic extract of some herbs such as Rosemarinic acid and Altheae officinalis, an increase in dermal pailla cell proliferation of human hair follicle was observed. Consistent with the previous study, another study showed that ethanolic extract of Rosemarinus officinalis and Altheae officinalis have positive effects on viability and proliferation of dermal papillae cells of human hair follicle; based on this study these herbal extracts have significant effects on expression of CyclinD1, Cdk4, Erk, Akt, Bcl-2, and Bax proteins in cell culture of human dermal papillae. It is suggested that activation of Akt and Erk pathways lead to these results or maybe it is due to increase in expression of Cdk4 and CyclinD1 (16), which are the checkpoints of G1/S as the regulator of cell cycle (18); the role of signaling pathway of Akt on mitogenesis and cell growth is previously showed (19) on the other hand and may be due to the fact that antioxidant content of such herbal extracts helps to save the cellular energy. Therefore the cell can focus on its vital activity such as proliferation and mitosis, gene expression, and transcription instead of fighting to free radicals while it is previously showed that Akt has a key role on cell viability signals (20, 21).
4.1. Conclusions
These results suggest that herbal extract may produce positive effects on the human dermal fibroblast cells. Overall, we have demonstrated the anti-cellular senescence effect of herbal extracts, suggesting that herbal extracts may be a good candidate for anti-aging formulas in comparison with modern cosmetics approaches.