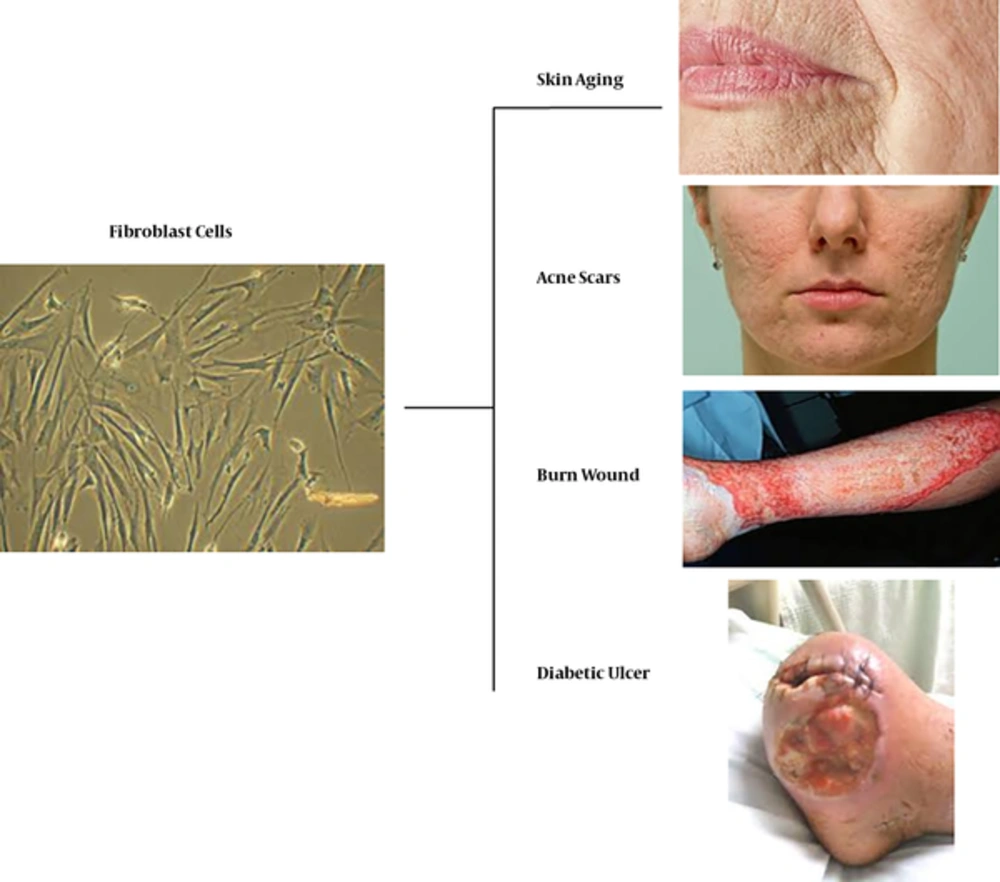
1. Context
Cell therapy with autologous dermal fibroblasts shows remarkable potential and is currently achieved by different methods such as spraying, grafting, and injecting to treat various types of ulcers, wrinkles, acne scars, and surgical wounds (Figure 1).
2. Evidence Acquisition
2.1. Application of Fibroblasts in Burn Wounds
The progress made in the care of patients with resuscitation and nutrition has improved the survival of individuals with extensive burns. However, extensive burns still cause many problems for both patients and physicians, causing mortality and morbidity of a large number of affected individuals (1, 2). Wound care methods in burn patients have changed tremendously in recent decades, resulting in improved mortality and morbidity. One of these methods is the use of split-thickness skin graft technique (3), which uses a portion of the patient’s healthy skin to cover burned areas. Unfortunately, this technique is ineffective in cases of massive burns and when healthy skin is not readily available. However, this treatment is still the preferred and standard principle to cover smaller burns (4). Over the past two decades, keratinocytes have been successfully used for the treatment of third-degree burns and transplantation with such cells can increase survival of patients (3, 5-7). The use of keratinocyte cultures provides excellent results when keratinocytes are transplanted onto a prepared wound bed. The amount of keratinocyte cells remaining on the wound bed, depending on bed conditions and other factors, reaches 15.55% (5, 8). For example, when these cells are transplanted onto a chronic granulation tissue, 15% of live weight is observed, and when they are transplanted on a newly or recently removed granulation tissue, 28% - 47% shows successful transplantation and 75% - 45% of tissues survive if affected areas are already covered with dead skin (9). On the other hand, in the first few weeks after the burn and when keratinocyte proliferation remains insufficient, the risk of mortality is very high due to complications, such as water disorders and electrolyte, other burns, and infections. Considering the above two conditions, using a technique in which the patient’s burn wound can be adequately covered is necessary. With the use of keratinocyte culture as a major step for burn patients, studies have shown that adding fibroblasts to cell cultures has improved wound healing both in terms of function and aesthetics. In addition, it showed wound closure and healing (10). The derm protects wounds against contraction and prevents scar formation. In fact, its absence may result in the immediate rejection or delayed ligation and contraction of wound and non-consolidation of transplanted cells (4). Although the use of keratinocyte cultures has been proven to be effective in the treatment of burns. The addition of fibroblasts to cultured cells will result in better repair of ulcers in terms of efficacy and aesthetics (10).
In a method described by Hansbrough et al., the combination of separate and parallel cultivation of keratinocytes and autologous membrane-free fibroblasts of collagen-glycosaminoglycans (C-GAGs) has been produced. The structure of C-GAG membrane has been completed in terms of pore size, which allows fibro-vascular tissues to grow from the wound bed while retaining keratinocytes at its surface. A non-porous surface of C-GAG is drawn on the membrane to provide a smooth surface for cultured keratinocytes (11).
In one method, a scaffold was constructed using dispersed collagen, resulting in well-grown fibroblasts. This method was developed by Lie Ma et al., and uses lysine amino acid to prevent collagen degeneration. Collagen was prepared from glycine, glutamic acid, or lysine adjacent to water-soluble carbodiimide, 1-ethyl-3-dimethylaminopropyl-carbodiimide (EDAC), and hydroxysuccinamide and caused no cyto-compatibility problem. Indeed, these fibroblasts and collagen can be well transplanted onto humans in the desired position (12). In another method used by Glushchenko et al., in 1993 and 1994, cultured fibroblasts were used to treat grade III B and A burns and were remarkably successful in areas with limited burns (13). In a method reported by the same individuals in 1994, the healthy skin of burnt patients was used for auto-graft transplantation; the mesh was prepared with the diameter of each hole 6 times that in previous methods.
The mesh was then placed on the burned areas and fibroblasts were sprayed onto these wounds. After a week, the fibroblasts multiplied and wound edges were rapidly recovered. This method was successfully performed in 184 patients.
In a method reported by Lee SB et al., scaffolds of gelatin and beta-glucan with holes measuring 90 - 150 µm in diameter were prepared a/.nd used to construct a three-dimensional skin, fibroblasts, and keratinocytes. On the scaffold prepared from gelatin, β-glucan, and EDAC hydrochloride, the autologous culture was added, and in vivo studies showed that after a week, the transplanted skin was completely restored (14).
In a method reported by Liames SG and colleagues, a person’s own plasma cluster was used as the matrix in which human fibroblasts were cultured. Then, human keratinocytes were placed on this scaffold, and after 24 - 26 days, these keratinocytes increased by 1000 times. Later, the three-dimensional skin was transplanted on two burned patients; after 2 years, excellent results were achieved (15).
2.2. Application of Fibroblasts in Diabetic Foot Ulcer
A foot ulcer, in diabetic patients, is one of the main problems caused by the inappropriate response to various treatments. These ulcers are progressive and highly resistant to micro-vascular involvement in diabetes. Chronic wounds are wounds that have not improved for 4 - 6 weeks. These lesions show delayed or no recovery. Given the presence of infectious or gangrenous tissues, no alternative is available except for amputation to prevent progression of lesions. Losing a leg or part of it is a serious problem for patients, both psychologically and socially. Thus far, various ways have been used to treat or to reduce complications of these ulcers. These methods included surgical and chemical debris, autograft and allograft bonds, use of synthetic grafts, which has advantages and disadvantages. Nowadays, with the advancement of science in the field of modern tissue engineering and widespread cell proliferation in laboratory conditions, these technologies can be possibly used to repair chronic and resistant wounds as well as extensive skin lesions, which are unlikely to be used for autologous tissues.
The diabetic foot ulcer is a condition where skin cells have lost the ability to replicate and repair. Previous studies have shown that transplantation of keratinocytes and fibroblasts from healthy areas of the skin increases cell proliferation and wound healing in affected areas. This method is less costly than other synthetic grafts, which are less likely to be infected than synthetic and non-synthetic grafts. Besides, it is less time-consuming than cell culture on a scaffold. The use of autologous cells in this method also reduces chances of transplant rejection.
In patients with cutaneous dysplasia, repair is the most difficult stage of healing cellular wound and matrix. As the closure of wound surface with epithelial cell augmentation is an important stage in the repair process, considerable effort has been exerted to produce and replicate epithelial cells in laboratories (3). In fact, cells that were used originate from autogenic, allogeneic, or xenogeneic lines. Tissue engineering techniques utilize somatic cells, which include epithelial cells (keratinocytes), fibroblasts, or a combination of these two cells (16, 17).
Fibroblasts exhibit different activities during repair. Initially, these cells migrate from adjacent tissues to wound areas. After that, fibroblasts multiply and produce an enriched collagen-rich extracellular matrix that covers the wound area. In addition, these cells produce growth factors that increase proliferation and production of keratinocytes in the body. Infiltration of fibroblasts in damaged areas can help with the secretion of materials and proteins to re-create and normalize the dermal layer. Therefore, these cells can help reduce the effects of scarring and enhance quality and appearance of the skin. However, in addition to fibroblasts, keratinocytes, as the main epidermal cells, play a significant role in developing the outer skin layer. Therefore, after infiltration of fibroblasts and preparation of a suitable substrate, keratinocyte cells are used to obtain results closer to the conditions of natural skin (18).
Several methods are used for the treatment of skin lesions, and one of them is skin grafting. The advantages of using this method include the rapid closure of the wound surface, which prevents invasion of microorganisms. This method requires the construction of a new texture. Other methods include using skin substitutes, such as cell-free matrices, which will cause fibroblast migration to lesion sites and can be used permanently or temporarily on wounds. Another method is the proliferation of small tissue epithelium that is autologous with tissue culture. The new method involves the use of collagen with fibroblasts as the underlying dermal layer, which is coated with a layer of keratinocytes as the epidermis. This skin substitute contains connective tissues and living cells (19).
In this area, studies have been conducted over the past decade and yielded promising results. An article, in 2002, showed that fibroblasts and cultured keratinocyte grafts caused complete wound healing in 60 days and exhibited no recurrence in the 16-month of follow up (17).
In a study by Curran et al., in the year 2, keratinocytes and human fibroblasts cultured on collagen type 1 were used for the treatment of diabetic foot ulcers. After 6 months, the percentage of improvement in patients using this method reached 63% versus 40% of controls, and the average time to close the wound surface totaled 61 days versus 181 days of the control group (20). The accepted role of these cells in wound healing is remarkably significant to that in new tissue engineering studies. One perspective considers an appropriate scaffold for transportation of these cultured cells, which are also sprayed to the wound surface, whereas some consider genetic intervention. Both two cell types produced are used to create a viable skin (21).
According to a study conducted by Svensjo et al., in 2002, in Sweden, wound healing was performed on diabetic patients; keratinocytes and autologous fibroblast cells were cultured in separate groups and in wounds belonging to the same group. Results showed the better rate of recovery and re-epithelialization in groups injected with fibroblast than those of the control group. Findings also showed that 8 days after injection, 17% of the wounds of the combined group of fibroblasts and keratinocytes were completely re-epithelialized, whereas 0% was observed in keratinocyte-only, fibroblast-only, and control groups. The number of cellular colonies in the wound group, which was injected simultaneously, was twice that in the keratinocyte group. In fibroblast and control groups, the number of cellular colonies was also extremely small (22).
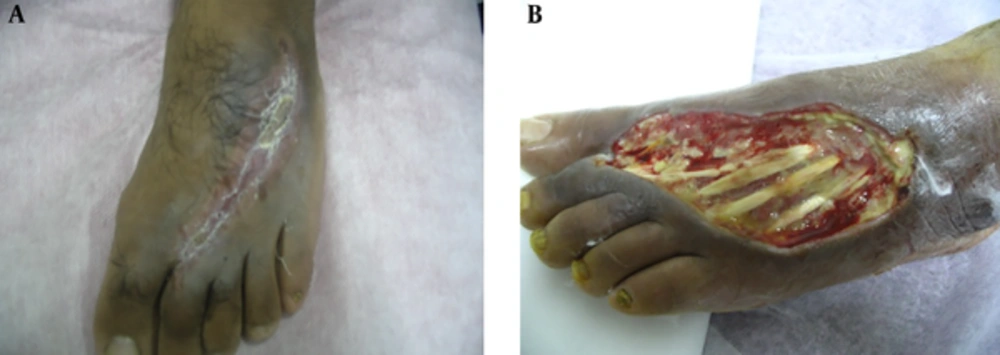
Velander et al., injected keratinocytes and fibroblasts into deep bumps on the back of the animal during a 2009 study on diabetes mellitus in the same university. On the 12th day after injection, the percentage of re-epithelialization in the fibroblast group reached 86.75%, 91.3% in keratinocyte group, and 56.8% in the control group treated with normal saline. On day 14, complete repair of keratinocytic wounds and the epidermal layer was observed in 59% of the control group (23, 24) (Figure 2).
2.3. Use of Fibroblast Cells in Scar Treatment
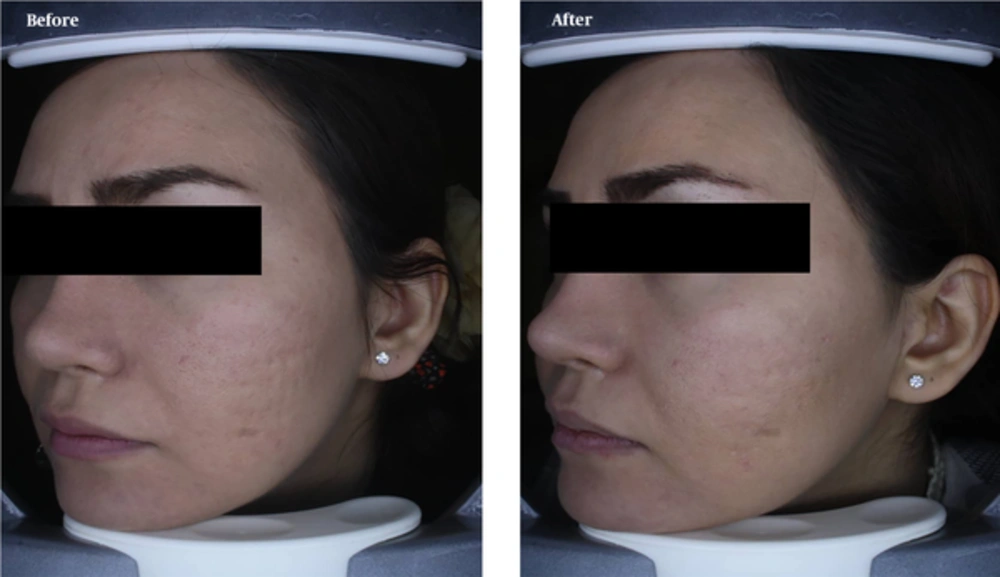
Acne vulgaris is one of the most common skin diseases and is the most common cause of referrals to dermatologists. To treat acne scars, fibroblastic cell transplantation is a relatively stable therapeutic approach. As fibroblasts are the main cells in repairing wounds and skin scars, their injection can be used for the treatment of patients. In this method, the biopsy is first prepared from the skin of the patient, and fibroblastic cells are then cultured. After replication, the cells are injected into the subcutaneous area in the scar regions (25). In this regard, Leishmaniasis is a disease caused by a leishmaniasis protozoan parasite. One of the main problems of patients after medical treatment and elimination of parasitic infection is the replacement of bad scars in visible areas in the patient. These scars are deep and can measure 2 - 3 cm in diameter, which in many cases can cause leprosy in patients. The main cause of these scars is the destruction of skin structure in the affected area. Therefore, cell therapy with fibroblastic cells and keratinocytes in these patients can be curative and lead to scar repair. In this method, after obtaining biopsy samples from a patient’s skin, fibroblastic cells are cultured and injected several times in the scar position. For this treatment, transplant of fibroblastic cells can be used in the lesion area only as a single injection of cells along with filler materials, such as hyaluronic acid or collagen (Figure 3) (26).
3. Results
Recent developments in the field of cell science have been dramatically improved. One of the most important parts of these developments is the emergence of a cell-therapeutic approach, in which cells are used to treat a wide range of diseases. Research on cells is undoubtedly one of the most interesting and controversial cases of medical science (27). Fibroblasts can be artificially grown and multiplied in laboratory environment via cell culturing and also being trans differentiated into specialized cell types such as muscles, nerves, or epithelial cells (28). Over the years, various studies carried on applying of fibroblasts in wound healing that their successful outcome is a proof for their commercial use.
4. Conclusions
Appling fibroblasts as the main cell source in skin results in a dramatic and rapid improvement in the function of affected in this organ (29, 30). On the other hand, skin diseases around the world rise with the increase in accidents, changes in people’s lifestyle habits, and other diseases that affect the skin. In the meantime, given the lack of donor and safety problems for aggressive actions, skin grafting is the best replacement option for these activities (31). Therefore, treatment with fibroblastic cells, in an appropriate biomaterial matrix, can improve their efficacy. These cells can actively affect the same cells or neighbor cells when exposed to any environment by specific secretion and expression of their differentiating markers (32). Extensive research on the proper function of these cells for treatment of ulcers, diabetic ulcer, skin wrinkles, and burns (33) have proven to be promising. Therefore, cell therapy, based on autologous dermis fibroblasts, provides good treatment for skin regeneration (34). Therefore, as cellular domain is one of the most important areas for commercialization of knowledge, a platform to develop science and technology in the field of supply and processing of stem cells is needed. Especially in the treatment of skin lesions with cells, development of clinical guidelines and infrastructure of relevant production lines can provide significant aid.