1. Background
Bacterial skin infections affected about 155 million people in 2013 (1). Some bacterial infections are mild and easily treated with topical antibiotics; however, others require an oral antibiotic (1). Staphylococcus aureus (S. aureus) is a Gram-positive bacterium that can live as a commensal organism on the skin, nose, and throat (2). S. aureus is a widespread cutaneous pathogen responsible for the great majority of bacterial skin infections in humans (3). Staph infections are the most often that affect the skin. They can go away on their own, however, sometimes they need to be treated with antibiotics. Antibiotic resistance is now recognized to be a serious hindrance to the management of S. aureus (3). Methicillin-resistant Staphylococcus aureus increased in the past decade (4) and there is an urgent need for new strategies to tackle S. aureus infections (5).
Pseudomonas aeruginosa a species of Gram-negative bacilli belonging to the family Pseudomonadaceae, is a bacterium that causes a wide variety of infections that have characteristic skin manifestations (6). P. aeruginosa is the major cause of nosocomial infections and a problematic pathogen due to its low susceptibility to antibiotics (7). Infection with P. aeruginosa can be a serious complication of burns. In these patients the disruption of the tegument, combined with the local immunosuppression that comes with burnt tissue (reduced T-cell activity, inflammatory cytokines and complement), means colonization frequently progresses to infection. Furthermore, P. aeruginosa is a particular problem in these cases (8).
In the early 20th century, the advancement of chemistry led to the development of the pharmaceutical industry and the provision of chemical drugs. Since then, new chemical drugs have been made by scientists and marketed. Although chemical drugs than herbal drugs treated diseases faster and have a stronger effect, the adverse consequences of their consumption are more than herbal medicine. In addition, due to antibiotic resistance, today, many medical and pharmaceutical experts believe that patients should be directed to the consumption of medicinal herbs. In the same spirit, in the last decade, pharmacists have been making and marketing a herbal extract of various drugs (9). Pimpinella anisum, belonging to the umbeliferae family, is an aromatic plant, which has been used in Iranian traditional medicine (10). It has an antimicrobial (11), anti-fungal (12), anti-viral anti-oxidant (13), and muscle relaxant effects (14). In this in vitro study, effect of antibacterial activity of polar fraction of the Pimpinella anisum extract has been determined against Staphylococcus aureus and Pseudomonas aeruginosa. In 2003, Ates and colleagues conducted a study on the antimicrobial activity of Pimpinella anisum alcoholic extract. It was concluded that the alcoholic extract of this plant on Micrococcus luteus and Mycobacterium semgmatism show antibacterial properties (11). Shukla’s study (15) regarding the evaluation of antifungal agents in the main oil of Pipinella anisum showed that the main oil of this plant that has a significant anti-fungal effect is Anethol. Akhtar et al. (16), reviewed the effect of different fractions of Anisum extract on pathogenic bacteria. Aqueous, acetone, ethanol, and methanol were used to prepare the extract, and the effect of extracts on Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Klebsiella pneumoniae was investigated. The results showed that most of the effect was on the aqueous extracts and the least effect is on petroleum ether solvent. Chaudhry and Tariq (17) with an investigation into the antibacterial effect of black pepper extract and anisone extract on isolated isolates from the mouth, showed that both extracts had antibacterial properties and the highest effect of the Anisum extract is on the Micrococous rooseus bacterium.
2. Methods and Results
This research was carried out during the summer of 1396 in biochemical and microbiological laboratories of the faculty of veterinary medicine, Islamic Azad University, Tabriz Branch. In this experimental study leaf footstalk was used. The plant was collected and dried off from the sun and at room temperature, then pulverized by electric mill and the extract of Pimpinella anisum was prepared.
2.1. Prepration of Extract
Soxhlet method was used for extraction. In order to prepare polar extract of anisum, 300 gr of powder of anisum was poured into the filter paper wathman No. 42. In addition, a little Merk methanol was smeared and inserted into the Soxhlet. To the balloons attached to the Soxhlet, about 500 milliliters of pure methanol were added and then heated. In the next step, Heidolth rotary machine was used for pure extraction at 40°C under vacuum.
2.2. Preparation of Bacterial Strains and Microbial Suspension
Standard strains of bacteria were obtained from the microbiological collection of the institute of industrial research of Iran. In order to prepare the bacteria, the fluidophilic specimens were transferred to a fluid medium (BHI) and cultured at a temperature of 35 - 30°C for one night. After creating turbidity in a liquid medium, the samples were cultured on a solid culture medium (BHI) to ensure their purity. In order to prepare a microbial suspension, fresh and young bacterial colony were transferred to the broth Muller hinton broth and incubated at 37°C for 2 hours, therefore, the resulting opacity is similar to the opacity of the tube at 0.5 McFarland (1.5 × 108 bacteria per milliliter).
2.3. Determination of the Antimicrobial Properties of the Extract by Well Diffusion Method
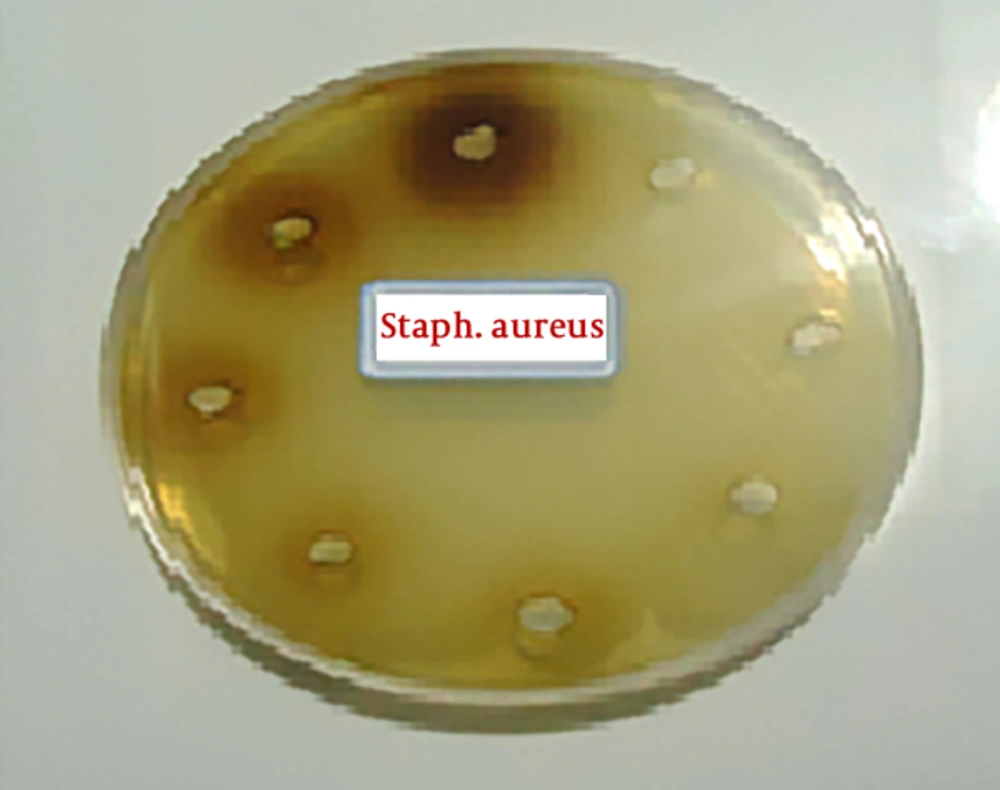
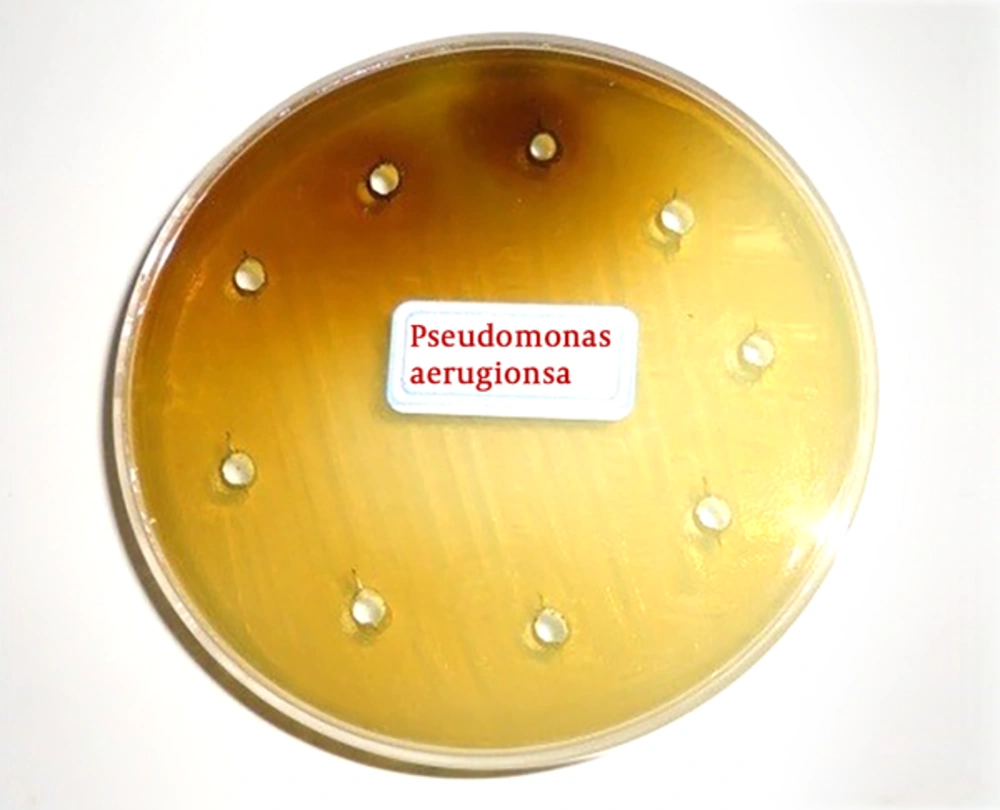
First, the extracts were diluted in 9 tubes then, 0.5 cc of DMSO solution with a concentration of 5% was poured into all tubes. Next, an amount of 1-cc of extract was poured into in the first tube, and after mixing, 0.5 cc was removed and transferred to the next tube. This action continued until the ninth pipe, and went from it to the outside. In this way, as indicated in Table 1, dilutions (0.39, 0.78, 0.56, 1.23, 25.6, 12.5, 25, 50, 100) of extracts were prepared. Next, in each tube, the 5-mm diameter blont disks were dispersed and after a half-hour, they were dried in a 37°C incubator. Afterwards, the bacterial culture was performed on a plate with a sterilized swap, and then the discs were placed at a plate level at a distance of 2 centimeters. As a positive control, antibiotic chloramphenicol was used as a negative control for dimethylsulfoxide DMSO. All cultures were incubated at 37°C for 24 hours. Furthermore, as shown in Figures 1 and 2, the bacterial cultures (Staphylococcus aureus and Pseudomonas aeruginosa, respectively) were evaluated for the formation or non-formation of the inhibition zone and the diameter of the inhibition zone in millimeters around the disk was measured by the caliper (the results are shown in Table 1). The inhibition zone diameter was an interaction of the concentration of the extract. By measuring the diameter of the inhibition zone and comparing it with the standard (Inhibition diameter created by chloramphenicol antibiotic), the antimicrobial activity of the extract is determined. An inhibition zone was observed for both bacteria. In the micro-plate method the maximum effect of extract was on Pseudomonas aeruginosa bacteria. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for this bacterium are 12.5 and 50 mg /mL. Polar extract of anisone plant, only at a concentration of 100%, infected on Staphylococcus aureus.
| Extract % Assay | 100% | 50% | 25% | 12.50% | 6.25% | 3.12% | 1.56% | 0.78% | 0.39% | Control | Medium Control | Extract Control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa PTCC 1430 | ||||||||||||
| MIC | - | - | - | - | + | + | + | + | + | + | - | - |
| MBC | - | - | + | + | + | + | + | + | + | |||
| Well | 10 | 10 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Staphylococcus aureus PTCC 1683 | ||||||||||||
| MIC | - | + | + | + | + | + | + | + | + | + | - | - |
| MBC | - | + | + | + | + | + | + | + | + | |||
| Well | 10 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
The Results of MIC/MBC and the Diameter of the Inhibition Zone in Millimeters in Different Concentrations of Polar Extract by Well Distribution Method
2.4. Determine the Minimum Inhibitory Concentration of Extracts by Microtiter Plate
Microtiterpolyt method was used to determine the minimum inhibitory concentration of the extracts by using the reductase of Rezazurin produced by the Aldrich Company. In this method, 96 well microplates were used. The well number 1 to 9 are about the dilution of 100% to 39% of the extract. The well number 10 is a bout bacteria, number 11 is control of the culture media and the well 12 was considered as the control of the extract. The range of color variations of Rezazurin is from blue (pH > 6.5) to bright red color (pH > 3.8).
2.5. Method for Determining the Minimum Inhibitory Concentration of Extracts
In each pit, 100 μL of the culture medium of the Muller Hinton Broth was poured except for the first pit. In the first and second pit, 100 μL of the extract was prepared (as the concentration of extract in the first pit is 100%), then 100 μL was transferred from the second pit to the third pit and from the third to fourth and to pit 9. From pit 9, 100 micro liters was released. In order to reach the pathogenic dose, from the 24 hour culture of the bacteria, 1 to 100 dilution was made and 100 μL was poured in all the pits and 10 μ Razazorin was injected to all pits. At this point, the color of all the pits became blue. The pit No. 10 was a bacterial control, and the growth of the bacterium is seen pink. The pit No. 11 was a culture medium control; the growth of the bacterium in this pit should be negative and its color violet. No. 12 is an extract control and is used to control the extract in terms of contamination. The color purple indicates the sterility of the extract and the lack of growth of the bacteria. After 24-hour incubation, the pit were examined about color variation of Rezazurin from blue violet to pink, which caused by the growth of bacteria. The lowest dilution of the extract, which the color change was not observed, the minimum inhibitory concentration of this extract was considered.
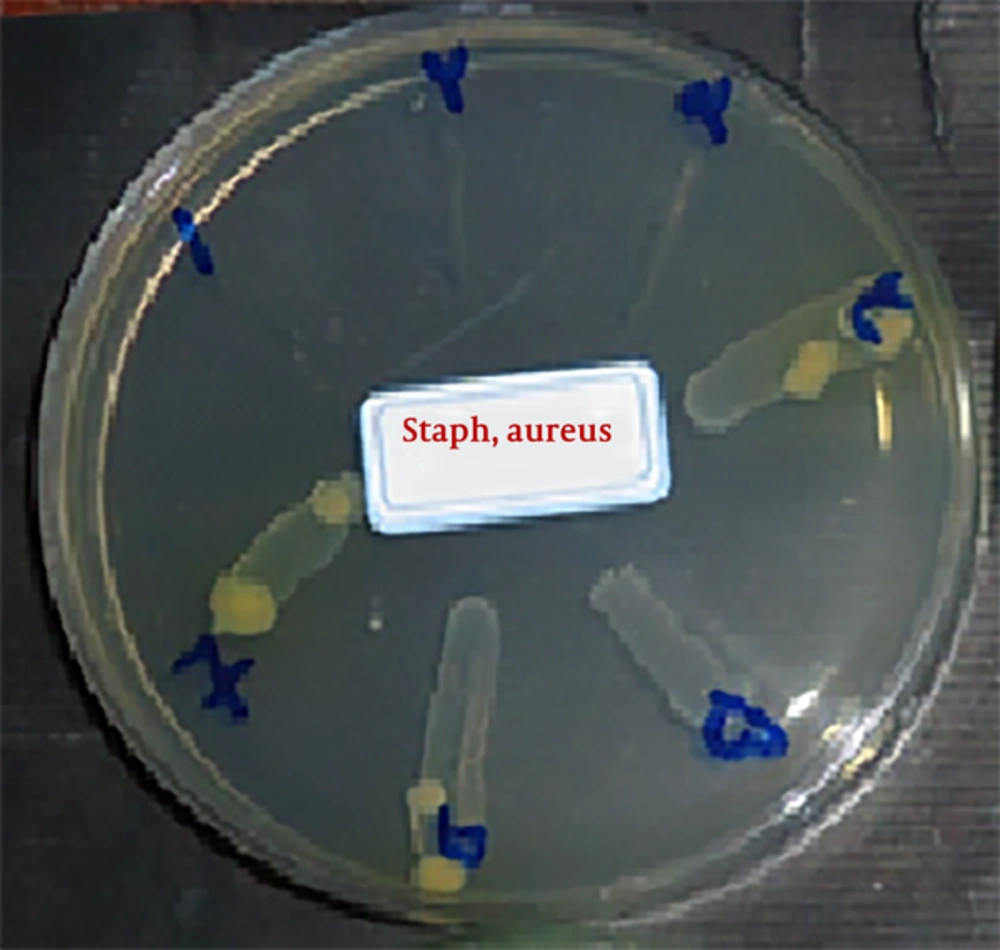
2.6. Determination of Minimum Bactericidal Concentration
To determine the minimum bactericidal concentration of extracts from the pit, which is considered as the minimum inhibitory concentration of bacterial growth, together with two wells before and two wells after, the specimens were removed and cultured in the Muller Hinton agar medium, as shown in Figures 3 and 4. After 24 hour incubation the growth of bacteria (Staphylococcus aureus and Pseudomonas aeruginosa, respectively) was evaluated. The plate related to the tube containing the lowest concentration of extract and no bacterial growth was observed, or considered as MBC.
2.7. Statistical Calculation
These tests were performed for extract and each bacterium was tested 3 times. In the end, the data were tested by Chi and Fisher test with significant level (P < 0.05). In addition, it was analyzed by SPSS software version 21.
3. Discussion
The skin is the largest organ of the body. Its function is to protect the body from infection. Sometimes the skin itself becomes infected. It is an important cause of morbidity and mortality among hospitalized patients and a major therapeutic challenge for clinicians. Effective management of skin infection includes systemic antimicrobial therapy. Herbal extracts have been used for the treatment of diseases since ancient times. Considering the compatibility of these substances with the body and its beneficial effects, research on the antibacterial effects of plants that have been reported in traditional medicine for the treatment of diseases is valuable (18). One of these herbal plants is Pimpinella anisum, it is a grassy, flowering plant with a height of 30 - 50 cm, and it has very fragrant beans, which is native to the Eastern Mediterranean and Southwest Asia. It grows in the east and northwest regions of Iran (10). Due to the emergence of antibiotic resistance as one of the problems and approach of communities is to increase the use of natural substances, This study was conducted to determine the antibacterial effect of Pimpinella anisum polar extract on standard strains of Pseudomonas aeruginosa and Staphylococcus aureus by well diffution agar and microtitrplate methods. The antimicrobial effect of the anisum extract is due to the chemical composition of the plant. The most important compound in the anisum responsible for its taste and aroma is a phytoestrogen compound called anethole (18). The isolation and structure of the flavonoids of anisum by cellulose column chromatography method led to the isolation of quercetin 3 gluconide, routine, luteolin 7 glucoside, isoerythrin, as crystalline and epinephrine glycoside compounds, and luteolin glycoside as non-crystalline compounds (19). Some of these chemical compounds have antibacterial effects, including β-caryophyllene, which has antibacterial properties (20). In addition, the comarins are antibacterial and even anti-cancerous and Flavonoids, such as Epigenin and Isovitexin, are among the most potent antimicrobial agents (20). In this study, Pimpinella anisum had a high inhibitory effect on Pseudomonas aeruginosa and had an inhibitory effect on Staphylococcus aereus in 100% concentration; this is probably due to the anethole, betacorifylin, and flavonoids. The study of Al-bayati, on antibacterial synergistic effects of methanolic extracts and essential oils of clove and anisum on nine bacterial species showed that the extracts of essential oils and methanol of these plants had inhibitory effects on most of the tested bacteria. The combination of methanolic extracts and major oils has synergistic effects on most of the tested bacteria. The extract of the present study is consistent with the inhibitory effects of Al-bayati study extracts on inhibitory effects on both bacteria tested (21). The study of Gulcin et al. (22), at 2003, regarding the antimicrobial effects of and aqueous and methanolic extract of anise against 10 species of bacteria and Candida albicans with disk diffusion showed that methanolic extract of Anisum have inhibitory effects on all tested bacteria, however, it did not affect the Candida albicans. The aqueous extract had no inhibitory effects on gram-negative bacteria; however, it showed inhibitory effects on Candida albicans. The antimicrobial effects of methanolic extract in this study are consistent with the inhibitory effects of the extracts of the present study. In the study of Gulcin et al. (22), anisone seeds were used, while in the present study, the anise extract from leaves and stems was prepared and does not conform to the current study. The Kermanshah study in (2009), the antimicrobial effect of Pimpinella anisum, and salvia was evaluated on Streptococcus mutans, Lactobacillus rhominosus, and Actinomyces viscose by Broth macrodilution. The results of this study showed that both extract had inhibitory effect on growth of all three bacterial species, which was significantly higher for Salvia than Anisum and in concentration range of 2 extracts had a bactericidal effect on all 3 bacteria. The present study is consistent with the test method and the inhibitory effects of the extracts on the bacteria studied (20).
3.1. Conclusion
According to the findings of this research, it can be concluded that the polar extract of Pimpinella anisum plant has antibacterial properties against the bacteria studied. Due to the variable results, use extracts, as a natural treatment of skin infection, requires further investigation.