1. Background
Endomyocardial biopsy (EMB) still remains the gold standard to assess the histopathological consequences in patients following heart transplantation (1). The sensitivity and high specificity of this method are significant for the diagnosis of acute cellular rejection (2, 3). Numerous studies are being investigated to replace this method with a non-invasive method or to increase the precision and accuracy of the biopsy, for example several cardiac MRI methods are being studied; as well as serum biomarkers and ECG; however, none of them, alone or even combined with other clinical symptoms, have been applicable as a replacement for periodic biopsy (4, 5). There is still no cardiac imaging or serum biomarker that could provide an appropriate replacement for histological assessment of cardiac biopsy in terms of survival and long-term stability of these patients (6).
Biopsy is valuable because it provides an initial assessment of the condition of myocardial damage, especially in terms of hypertrophy, ischemia, or the presence of any other pathological process, such as myocarditis (7). In the final correction made in ISHLT-WF 2004 (8), lack of lymphocytic inflammation was considered to be the marker of no acute rejection following transplantation, whereas the presence of mononuclear cells infiltration in the interstitial or perivascular region without disrupting the tissue structure was considered as a form of mild rejection. Moderate rejection is signified by the presence of two or more regions of mononuclear cell infiltration associated with myocardial damage. A severe acute or grade three rejection is indicated by myocyte injury often associated with polymorphonuclear inflammatory cells, also accompanied by edema and hemorrhage.
An important point is the use of immunosuppressive drugs called calcineurin inhibitors. In this regard, the role of cyclosporine (9) and Tacrolimus is quite prominent. With the goal of significantly reducing the toxic effects of these drugs, their dosage should be regulated and adapted for each patient. Monitoring of drug concentrations is the most important point in this regard. High doses of cyclosporine are sometimes recommended at 250 and 350 grams per liter, mainly in the 6 to 12 months after transplantation (8). In some studies, the survival and graft stability results were similar in cyclosporine doses of 250 to 350 grams per liter and 150 to 250 grams per liter, and thus it seems that the low dose of cyclosporine can also be safe and effective (10). The timing of the administration of these drugs was also of some concern. Some studies assessed the effects of early or delayed use of drug which did not differ between the two groups in terms of graft survival (11, 12). Some have also shown that not only the decrease in cyclosporine dose does not affect the survival of the transplant, but will also reduce the incidence of neoplasia following the transplantation (12, 13). Also, in comparison with the efficacy and toxic effects of cyclosporine and tacrolimus, similar effects of the two drugs have been observed in the survival of the organ transplant (14-16). Some studies have acknowledged that although the efficacy of the two drugs in the graft sustainability is quite similar, some complications such as nephrotoxicity, hypertension, hirsutism and hyperlipidemia were more prevalent in cyclosporine-receiving patients and diabetes, neuropathy and alopecia were more common in those on tacrolimus (17). Therefore, it is recommended that tacrolimus be given priority in patients with hypertension or hyperlipidemia. In addition, women and children may benefit more from tacrolimus (18).
To take advantage of these drugs, serum drug monitoring is essential for the best drug efficacy and maintenance of survival and tissue stability. It is necessary to examine the relationship between immunosuppressive levels and their effects on the results of endomyocardial biopsy.
Recently, it is shown that cardiac magnetic resonance imaging (CMR) can be useful to detect the rejection of heart transplantation. It is a non-invasive and safe method but its cost and the inexistence of experienced and professional experts in this field restrict the application of this modality (6).
2. Objectives
According to the above-mentioned facts, to take advantage of these drugs, serum drug monitoring is essential for the best drug efficacy and maintenance of survival and tissue stability. It is necessary to examine the relationship between immunosuppressive levels and their effects on the results of endomyocardial biopsy. Therefore, we aimed to evaluate cyclosporine and tacrolimus dose changes during post-transplantation biopsies and their association with endomyocardial biopsy grades.
3. Methods
This retrospective observational study was performed on 100 cardiac transplantation patients at Rajaie Cardiovascular Medical and Research Center (RCMRC), a tertiary care hospital for cardiovascular patients in Tehran, Iran from 2015 to 2016 who subsequently had endomyocardial biopsies to assess the graft stability. The reports were categorized in three intervals of biopsies performed up to one month after transplantation, one to six months and after that.
The biopsies were numerous and on clinician demands but were concomitant in same patients during short time interval. This shows the therapist’s concern not only about the correctness of the biopsy results and the pervasiveness of the degree of regression but also about previous technical biopsy failures.
The results were checked only by one skilled pathologist who prevented interobserver variation. However, in multiple cases it was confirmed by external controls. The study protocol was approved by the research board of RCMRC.
In the present study, patients were divided into two groups based on the drugs they were given: cyclosporine (n = 13) and tacrolimus (n = 87). The baseline characteristics including demographics, medical history, the time of transplantation and the type of immunosuppressive drug were all collected by reviewing the recorded files. All patients underwent serial EMB surveillance based on the protocols suggested by International Society for Heart and Lung Transplantation (ISHLT) (8). The relevant information of tissue samples including grades of rejection was also collected from patients’ medical records. In addition, the serum levels of drugs were also taken into account. The concentration of the drug was considered in this study two hours after the injection (C2). In all histopathological slides, grading was performed based on the ISHLT-WF2004 criteria (8). All the samples were assessed and reported by a single experienced pathologist.
3.1. Statistical Analysis
Normality of data was assessed using one sample Kolmogorov-Smirnov test. Data described as mean ± standard deviation (SD) for interval and frequencies and percentages for categorical variables. Comparison between groups were performed by independent sample t-test or one-way ANOVA model for interval and Pearson’s chi-square test (or Fisher’s exact test, as needed) for nominal data. P value < 0.05 was considered as statistically significant. IBM SPSS statistics 22 for Windows (IBM Inc., Armonk, NY) was applied for the statistical analysis.
4. Results
One hundred patients, mean ± SD age: 27 ± 13.6 years (range: 1 - 55 years), 66 men were observed. In terms of gender distribution, the prevalence of male sex in the two groups receiving cyclosporine and tacrolimus was 61.5% and 66.7% respectively with no difference between the two groups (P = 0.759). The mean age in patients in the two groups was 32.23 ± 7.45 years and 18.61 ± 18.2 years, respectively that was significantly higher in cyclosporine group (P value = 0.001).
4.1. Biopsy Grading in Cyclosporine Recipients
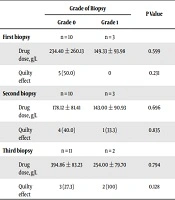
In 13 patients who were receiving cyclosporine, at the time of three consecutive endomyocardial biopsies, the prevalence of grades 1 was 3 (23.1%), 3 (23.1%) and 2 (5.4%) respectively. As shown in Table 1, no significant association between the biopsy grade and the mean plasma level of cyclosporine was found at the time of different biopsies. Also, the trend of the changes in the plasma level of cyclosporine within the follow-up time remained insignificant (P = 0.192). We could not reveal an association between the pathological grade in biopsy and Quilty effect either.
| Grade of Biopsy | P Value | ||
|---|---|---|---|
| Grade 0 | Grade 1 | ||
| First biopsy | n = 10 | n = 3 | |
| Drug dose, g/L | 234.40 ± 260.13 | 149.33 ± 93.98 | 0.599 |
| Quilty effect | 5 (50.0) | 0 | 0.231 |
| Second biopsy | n = 10 | n = 3 | |
| Drug dose, g/L | 178.12 ± 81.41 | 143.00 ± 90.93 | 0.696 |
| Quilty effect | 4 (40.0) | 1 (33.3) | 0.835 |
| Third biopsy | n = 11 | n = 2 | |
| Drug dose, g/L | 394.86 ± 83.23 | 254.00 ± 79.70 | 0.794 |
| Quilty effect | 3 (27.3) | 2 (100) | 0.128 |
aValues are expressed as No. (%) or mean ± SD.
4.2. Biopsy Grading in Tacrolimus Recipients
The results in the Tacrolimus group are summarized in Table 2. Regarding pathological grade related to endomyocardial biopsy, at the three consecutive biopsies, the frequency of grade 1 was 31 (35.6%), 19 (21.8%) and 14 (16.1%) and grade 2 in 3 (3.4%), 1 (1.1%), and 0 respectively indicating a downward trend in biopsy grade within the follow-up. In the first biopsy, the mean level of Tacrolimus in the grade 0 group was 9.57 ± 2.75, in the grade 1 group was 6.9 ± 1.87 and in the grade 2 group was 4.93 ± 1.11, indicating an adverse relationship between the biopsy grade and the serum level of drug (P = 0.001), however such an association was not found in the next biopsies (Table 2). The trend of dose changes in cases on Tacrolimus was completely significant after three consecutive biopsies, so that in the subsequent biopsies, the dose reduction was completely evident (P = 0.001). Similar to cyclosporine, we revealed no association between the pathological grade in biopsy and Quilty effect.
| Grade of Biopsy | P Value | |||
|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | ||
| First biopsy | n = 53 | n = 31 | n = 3 | |
| Drug dose, g/L | 9.57 ±2.75 | 6.59 ± 1.87 | 4.93 ± 0.11 | 0.001 |
| Quilty effect | 24 (45.3) | 18 (58.1%) | 2 (66.7) | 0.449 |
| Second biopsy | n = 67 | n = 19 | n = 1 | |
| Drug dose, g/L | 5.02 ± 3.14 | 5.89 ± 5.50 | 6.00 ± 0.01 | 0.530 |
| Quilty effect | 37 (55.2) | 6 (31.6) | 1 (100) | 0.117 |
| Third biopsy | n = 73 | n = 14 | n = 0 | |
| Drug dose, g/L | 5.64 ± 3.04 | 5.50 ± 1.83 | - | 0.871 |
| Quilty effect | 38 (52.1) | 6 (42.9) | - | 0.528 |
aValues are expressed as No. (%) or mean ± SD.
5. Discussion
Changes in the dosage of immunosuppressive drugs following organ transplantation, especially the heart, can be a potential predictor of long-term post-transplant outcomes. In this regard, it is claimed that the dose changes of these drugs may also be related to the degree of involvement in the histopathological evaluation of endomyocardial biopsy in the short term after transplantation. In other words, monitoring immunosuppressive drugs with the aim of improving the endomyocardial biopsy grade, which is a hallmark of transplant rejection, is very important, and optimizing the dosage of the drug in achieving a stable transplant is very effective. What we did in this study was to evaluate cyclosporine and tacrolimus dose changes during post-transplantation biopsies and their association with endomyocardial biopsy grade. What we found in this study was primarily the difference in the pattern of the relationship between the dose of drug and biopsy grade in the two drugs. In the case of cyclosporine administration, in different sequences after biopsy, there was no significant relationship between the plasma level of the drug and the grade of biopsy, while as for tacrolimus, there was a significant inverse relationship between serum tacrolimus concentration and biopsy grade the first time after transplantation (about one month after transplantation), although this relationship was not observed in the subsequent stages of biopsy. In the other hand, changes in serum concentration of tacrolimus may be an important indicator in the prediction of acute rejection. Although in long term, with the goal of evaluating and predicting chronic rejection, serum tracking of both medications to predict the graft rejection will not be effective. However, it should be considered that the dose adjustment of each immunosuppressive drug after long-term transplantation is necessary as it is necessary to monitor the dose of the drug needed to maintain a successful graft, as well as in controlling and preventing the direct or indirect side effects of the drug (susceptibility to infections and inflammatory diseases or organ damages). In some studies on the relationship between the serum level of cyclosporine and the risk of rejection, the drug adjustment proved to decrease rejection of the transplant. In this regard, for heart transplantation, achieving a cyclosporine dose greater than 850 ng/mL during the first week after transplantation led to a significant reduction in rejection rate (BAR-LEV) (19, 20). However, in some studies, there was no relationship between cyclosporine levels two hours after transplantation (C2) with biopsy grade. As in the study by Cantarovich et al. (21), there was no significant differences in C2 between the two groups with EMB grade 2 or less and with grade 3A or higher (22) INT. What seems to explain these differences is that various factors such as the type of immunosuppressive drug (based on our study), along with the administration of ATG or steroid drugs, the initial dosage of the drug, and the follow-up time, can be related to the association between drug dose changes and pathologic grade in endomyocardial biopsy. With that in mind, the lack of correlation between Quilty effect and changes in the biopsy grade was also important.
As a further point in our study, there was a significant decrease in the age of patients receiving Tacrolimus compared to cyclosporine. The choice of these drugs in addition to the effectiveness of suppressing immunity and the sustainability of the graft also affects the potential side effects of drugs, where drugs such as tacrolimus are superior to cyclosporine for women as well as for younger subjects. In general, it is shown that Tacrolimus seems to be superior to cyclosporine in patients with hypertension or hyperlipidemia and in younger patients. In addition, women and children may benefit more from tacrolimus (18). Also, in terms of aesthetics, the findings were in favor of tacrolimus consumption, rather than cyclosporine, and thus the tendency for administration of tacrolimus in younger patients was greater than cyclosporine (21).
In this study, we evaluated two commonly used medications used in our hospital, of course, there are patients who used drugs similar to this medication group, depending on the physician’s opinion, such as sirolimus and corticosteroids.
However, we did not include these patients in the study because of a small number of cases.
Also possible tissue agonist / antagonist effects of other drugs that are sometimes used in combination with the above drugs or in partial or sequential treatment, such as corticosteroids, etc.; are likely to be effective in biopsies and may be considered for future research.
5.1. Conclusions
As a general conclusion, an association between endomyocardial biopsy grade in the heart and the serum level of cyclosporine after transplantation is absent. But there is a significant reverse relationship between endomyocardial biopsy grade and serum tacrolimus concentration in the first weeks after transplantation and thus tracking and monitoring of serum tacrolimus after transplantation may play an important role in predicting acute rejection of transplantation. This may cause the medication to be changed or dose adjustment or new adjuvant treatment by the clinician. However, in the long term after transplantation, the above mentioned relationship may be meaningless by our data. However, it would be wise to maintain an optimal concentration of the drug within the normal therapeutic range.
5.2. Limitations of the Study
Due to the time axis of the study (retrospective data collection) and the inability of the researchers to interfere in the type of treatment, as well as the occurrence of missing data for various reasons, the number of cyclosporine-treated patients was clearly low, which may affect the study in this group. We should bear in mind this fact for future studies. In addition, cyclosporine may be used less in our hospital probably because of renal complications or cosmetic effects, then the sample size was limited during our study, so we could not find any significant association even though it may actually exist. Investigation of these cases requires more extensive studies and more samples to evaluate these issues that were out of the scope of this article.