1. Background
Breast cancer is not a simple disease. When we diagnose breast cancer, one of the first points that we should identify to manage the patient is the type of cancer. The breast cancer type is important for us to know how the cancer may behave and how to manage it (1).
The HER2 gene is present in about 15% - 30% of patients with breast cancer and 10% - 30% of patients with gastroesophageal cancers (esophagus, stomach and colon) and has also been seen in other cancers such as ovary, uterine, head and neck, bladder and lung (2).
Human epidermal growth factor receptor 2 (HER2) is a gene that makes proteins in the breast cell and this gene can control the healthy breast cell to divide, grow and repair itself. In HER2 positive breast cancers we have high levels of HER2 protein in malignant cells. In case of abnormal HER2 gene, breast cells may divide and grow at an uncontrolled rate, therefore it is expected that HER2 positive breast cancers tend to be more aggressive (1, 3).
Human epidermal growth factor receptor (HER2) targeted therapies include administration of trastuzumab, pertuzumab and ado-trastuzumab which have revolutionized the treatment of breast cancer, and the management of patients with trastuzumab in conjunction with adjuvant chemotherapy in surgically removed HER2-positive breast cancer may decrease the rate of recurrence and death (4).
Despite all the benefits of human epidermal growth factor receptor (HER2) targeted therapies, they can cause left ventricular dysfunction, hypertension, arrhythmia, disabling cardiac failure and cardiac death. The mechanism of HER2 targeted therapies is unknown. In clinical practice the most common side effect is left ventricular dysfunction especially when we use this group of drugs (HER2 target therapies) in conjunction with anthracyclines.
Some studies in which trastuzumab was used for breast cancer showed the risk of cardiac dysfunction and a decline in left ventricular ejection fraction (LVEF) ranging from 3% to 27% (5). Asymptomatic left ventricular dysfunction or heart failure occurs in up to 25% of patients managed with trastuzumab and in these patients the safety of continuing trastuzumab is unknown (6). Food and Drug Administration recommends, trastuzumab, pertuzumab and ado-trastuzumab usage for patients whose baseline cardiac function (LVEF) exceeds 50% - 55% and when the LVEF decreases during chemotherapy discontinuation of the treatment or dose delay should be considered (7).
The technique that we used in most cases for evaluation of cardiac function (EF) is echocardiography. Evaluation of Left ventricular ejection fraction (LVEF) by 2D echocardiography cannot detect subtle changes in LV systolic function which may be due to inadequate visualization of LV apex, regional wall motion abnormalities and inter-observer variability in measurements (8). Early diagnosis of subclinical cardio toxic effects due to anti-HER2 antagonists or anthracyclines may be considered as one preventive strategy.
An accurate and important method for evaluation of subclinical cardio toxic effects is myocardial strain imaging in which we can estimate myocardial dysfunction before overt heart failure (9). In fact a detectable drop in left ventricular ejection fraction, occurs only after the loss of a large amount of myocardial tissue, so a reduced ejection fraction after chemotherapy is often a sign of extensive myocardial damage (10, 11).
Nowadays we use 2D speckle tracking echocardiography to quantify longitudinal and circumferential strain parameters. Alteration in these parameters have been shown to precede decrease in LVEF (12).
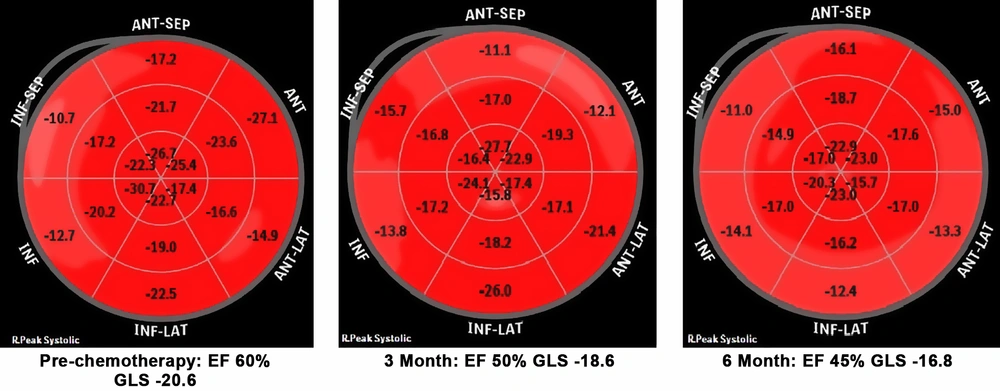
Regarding multiple studies, during chemotherapy an average of 10-20% decrease in GLS has been observed and a reduction of over 15% has had clinical significance (13).
In estimating LVEF, 3D echocardiography has an accuracy comparable to cardiac magnetic resolution (CMR) (11).
2. Objectives
In this study we want to draw a comparison between the two groups of breast cancer patients (HER2 positive and negative) by advanced echocardiography (2D, 3D echocardiography, GLS and GCS).
3. Methods
We have conducted a single center prospective study at Rajaie Cardiovascular Medical and Research Center. In this study we have evaluated all the patients who had breast cancer and were referred to our clinic before chemotherapy and every 3 months during chemotherapy (including Human epidermal growth factor receptor (HER2) targeted therapies and anthracyclines) in 2018 - 2019.
The abovementioned patients were examined by cardiology visits and transthoracic echocardiography. LVEF was assessed by 2D echocardiography, 3D echocardiography and heart model.
Exclusion criteria included patients who had uninterpretable echo images or prior heart disease, including history of cardiomyopathy, significant valvular heart disease and previous myocardial infarction causing ischemic cardiomyopathy.
Written informed consent was obtained from all patients in this study and it was approved by Iran University of medical sciences ethical committee.
All echocardiograms were performed following study protocol at baseline and every 3 months during treatment. Transthoracic echocardiography was performed for all patients. An ultrasound instrument (Epic7c, Philips medical systems) was used with an X5-1 transducer. All the measurements were performed by one observer in order to avoid inter-observer variability.
Left ventricular volumes and ejection fraction were calculated by 2D transthoracic echocardiography, 3D echocardiography and heart model. Two dimensional ejection fraction was calculated from left ventricular end diastolic volume and left ventricular end systolic volume in apical four chamber and two chamber views. We also calculated left ventricular end diastolic volume and left ventricular end systolic volume, stroke volume (SV = EDV-ESV), CO (CO = SV × heart rate) and EF (EDV-ESV/EDV × 100) by 3D echocardiography. Two dimensional global longitudinal strain was measured after recording 2 chamber, apical 3 chamber and apical 4 chamber views. 2D GLS was calculated as the average of 17 myocardial segments. For calculating global circumferential strain (GCS) short axis views at base, mid and apex were obtained (13).
All measurements were obtained blinded to patient’s history, dose of drugs and previous treatments.
All values are expressed as mean ± SD. Variable differences between before and after chemotherapy were analyzed using paired t-test or Wilcoxon signed-rank test. P values less than 0.05 were considered significant.
4. Results
This analysis included 58 patients with breast cancer with 4 follow-up echoes (pre-chemotherapy and every 3 months during chemotherapy). Fifteen cases (34%) were HER2 positive. Mean age in HER2 positive was 64 years (range: 45 - 69 year) and mean age in HER2 negative was 46 years (range: 45 - 55 range). Mean body mass index in HER2 positive patients was 26 (range: 25 - 34) and mean BMI in HER2 negative was 25 (range: 25 - 32). Among HER2 positive patients 2 patients (13.3%) had diabetes, 7 patients (46.7%) had hypertension, 3 patients (20%) had a history of ischemic heart disease, and 3 patients (20%) had valvular heart disease.
About 60% of HER2 positive patients had stage 3 and 74.3% of HER2 negative patients had stage 2 cancer. Among HER2 positive patients, 46.7% had grade 4 and 51.4% of HER2 negative patients had grade 2 cancer.
Mean left ventricular ejection fraction (2D LVEF) in HER2 positive patients was 55 % (range: 42.5 - 55) at baseline and in HER2 negative patients was 55 % (range: 52.5 - 55). In HER2 positive patients we had 10 percent decrease in LVEF during follow-up and the final LVEF was about 45% (P value < 0.05) (Table 1).
| HER2- | HER2+ | P Value | |
|---|---|---|---|
| EF2D(1) | 55 (52.5 - 55) | 55 (42.5 - 55) | 0.120 |
| EF2D(2) | 55 (50 - 55) | 45 (40 - 45) | < 0.05 |
| EF2D(3) | 50 (50 - 55) | 45 (38.75 - 45) | < 0.05 |
| EF2D(4) | 50 (50 - 50.5) | 45 (40.45) | 0.655 |
| EF3D(1) | 55 (50 - 59.5) | 57 (52.4 - 59.6) | < 0.05 |
| EF3D(2) | 52 (50 - 55) | 40 (39.75 - 46.25) | < 0.05 |
| EF3D(3) | 50 (50 - 55) | 40 (38.7 - 45) | < 0.05 |
| EF3D(4) | 50 (50 - 55) | 45 (40 - 45) | < 0.003 |
| EFHM(1) | 60 (60 - 61.5) | 55 (50 - 59.75) | 0.918 |
| EFHM(2) | 50 (50 - 55) | 50 (50 - 55) | < 0.05 |
| EFHM(3) | 55 (50 - 55) | 50 (50 - 55) | < 0.05 |
| EFHM(4) | 50 (50 - 55) | 50 (50 - 50.5) | < 0.05 |
aValues are expressed as median (IQR).
Mean left ventricular ejection fraction by 3D echocardiography (3D LVEF) in HER positive patients was 57% (range: 52.4 - 59.6) and in HER2 negative patients was 55% (range: 50 - 59.5) at baseline. In HER2 positive patients we had about 20% decrease in 3D LVEF and the final LVEF was 40% (P value < 0.05) (Table 1).
Mean left ventricular ejection fraction by heart model in HER2 positive patients was 55% (range: 50 - 59.75) at baseline and in HER2 negative patients was 60% (range: 60 - 61.5), with a 5% decrease in HER2 positive patients (P value < 0.05) (Table 1).
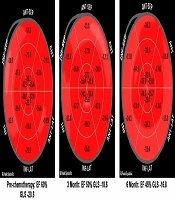
Mean global longitudinal strain (GLS) in HER2 positive patients was -18 (range: -17.2 to -21.6) and in HER2 negative patients was -19 (range:-18 to -20.8) at baseline which decreased to -18 in HER2 positive patients and -17 in HER2 negative patients, showing no clinical significance (P value: 0.146) (Table 2).
| GLS(1) | GLS(2) | GLS(3) | GCS(1) | GCS(2) | GCS(3) | |
|---|---|---|---|---|---|---|
| HER2+ | -18 (-17.2, -21.6) | -17 (-16, -18) | -17 (-15.25, -18) | -21 (-19, -23) | -17 (-16, -18) | -17 (-15, -18) |
| HER2- | -19 (-20.8, -18) | -18 (-17, -20) | -18 (-16, -19) | -21 (-20, -22) | -18 (-17, -20) | -18 (-16.5, -19) |
| P value | 0.182 | 0.146 | 0.045 | 0.005 | 0.008 | 0.003 |
Mean circumferential strain (GCS) in HER2 positive patients was -21 (range: -20 to -22) and in HER2 negative patients was -21 (range: -19 to -23) at baseline which decreased to -18 in HER positive patients and -17 in HER2 negative patients, showing clinical significance (P value: 0.008) (Table 2).
We also evaluated changes in LVEF, GLS and GCS during follow-up echoes by Friedman’s test in each group. The range of GLS and GCS showed significant changes during follow-up (P value < 0.05). We also monitored 2DLVEF, 3DLVEF and HMLVEF, which revealed that the range of LVEF significantly changed and decreased (P value < 0.05) during follow-up (Tables 3 and 4).
| Mean range in HER2- | Mean range in HER2+ | |
|---|---|---|
| EF2D(1), EF3D(1), EFHM(1) | 3.36, 3.9, 3.6 | 3.88, 4, 4 |
| EF2D(2), EF3D(2), EFHM(2) | 2.93, 2.3, 2.2 | 2.25, 2, 2 |
| EF2D(3), EF3D(3), EFHM(3) | 2.14, 1.9, 2.1 | 1.81, 1.71, 1.74 |
| EF2D(4), EF3D(4), EFHM(4) | 1.57, 1.9, 2.1 | 2.06, 2.29, 2.28 |
| P value 2D, P value 3D, P value HM | 0.009, 0.019, 0.130 | 0.001, 0.002, 0.001 |
| HER2- | HER2+ | |
|---|---|---|
| GLS(1), GCS(1) | 1.21, 1 | 1.23, 1.02 |
| GLS(2), GCS(2) | 2.16, 2.34 | 1.88, 2.12 |
| GLS(3), GCS(3) | 2.63, 2.66 | 2.88, 2.80 |
| P value GLS, P value GCS | < 0.05, < 0.05 | < 0.05, < 0.05 |
5. Discussion
We had 58 patients in our study, of which 15 (about 34%) were HER2 positive. These patients were examined by echocardiography at baseline and every 3 months during chemotherapy, up to 4 follow-ups. HER2 gene in normal breast cells causing growth and repair of the cells. Mutated HER2 gene lead to abnormally high levels of HER2 protein which caused abnormal cells to grow and divide out of control. HER2 positive breast cancers are more aggressive than other types, and the patients may have left ventricular dysfunction and lower ejection fraction.
In our study the mean age in HER2 positive patients was 64 years and in HER2 negative patients, 46 years, which showed a significant difference (P value = 0.018). HER2 positive patients were older than HER2 negative patients.
In our study, patients with previous cardiomyopathy were not included, 7 patients had diabetes, 17 patients had hypertension, 5 patients had ischemic heart disease and 6 patients had valvular heart disease. All of these risk factors were lower in HER2 positive patients.
We expected HER2 positive breast cancers to be more aggressive than other types. In our study most of the HER2 positive breast cancers were stage 3 (60%, P value < 0.05) and grade 4 (46.7%, P value < 0.05), which was significant, showing that HER2 positive breast cancers are more aggressive than HER2 negative breast cancers.
In our study HER2 positive breast cancers showed about 10% drop in 2DEF with a final LVEF of about 45%, about 20% drop in 3DLVEF with a final LVEF of about 40% and about 5% drop in HMLVEF with a final LVEF of about 50%, which were all significant (P value < 0.05).
The decline in HER2 positive patients may be due to treatment with trastuzumab in HER2 positive breast cancers. The second explanation is that advanced age (> 50 years) is more associated with trastuzumab cardiomyopathy, and in our analysis the mean age of HER2 positive breast cancers was 64years. We found that HER2 positive breast cancers are more aggressive and showed more decline in LVEF by 2D and 3D echocardiography.
Early cardiotoxicity may be silent and prompt diagnosis is important for these patients, therefore early diagnosis and appropriate therapy can decrease progression to clinical heart failure. In our study we measured global circumferential strain (GCS) which represents shortening along the circular perimeter and we also measured global longitudinal strain (GLS) that represents longitudinal shortening from the base to the apex. In this analysis we observed that both GLS and GCS decreased during chemotherapy especially in HER2 positive patients, but the results suggest that the drop in GCS was earlier and significant (P value < 0.05), while the drop in GLS was not significant in our study (P value = 0.146) (Figure 1).
We also evaluated changes in LVEF in each group during follow-up. The mean range in 2D EF, 3D EF, HMEF, GLS and GCS in HER2 positive breast cancers decreased during chemotherapy, which was significant (P value = 0.001).
GLS and GCS can detect subclinical involvement of cardiac function. We found that GCS is more sensitive than GLS in detecting subclinical involvement, and early changes in GCS is a good predictor of subsequent development of drugs (anthracycline-trastuzumab) induced cardiotoxicity.