1. Background
Congenital heart disease (CHD) is the most common congenital disorder in the neonate, accounting for 28% of all congenital anomalies. The disease is due to the lack of normal development of the heart during embryonic development (1). The prevalence of CHD worldwide is reported to be between 10 and 8 per 1,000 live births, however the prevalence is different in various regions (2). Improvements in diagnostic modalities, most importantly echocardiography, has resulted in an increased prevalence of CHD in Iran during the recent decades (3). Despite remarkable improvements in diagnosis and medical care, congenital heart disease still remains a major cause of infant mortality, causing considerable personal and social burden (4, 5).
Many factors are associated with the occurrence of CHD, including genetic factors, teratogenic exposure and several other unrecognized factors. Genetic abnormality is the leading cause of cardiac defect in a small proportion of these patients with CHD. Other associated factors, which are controllable and modifiable, have remained unrecognized. It has been demonstrated that 30% of CHDs can be prevented by identification of the modifiable factors and performing appropriate intervention, in this regard (6, 7).
Teratogenic factors (including maternal smoking, alcohol use, exposure to air pollution, thalidomide, vitamin A, retinoid, indomethacin, tocolitics and maternal infection with rubella) are associated with the occurrence of the CHD. Additionally, parental age, maternal history of epilepsy, mood disorders, phenylketonuria, diabetes type 1 and 2 are several other risk factors of CHD (7). Many actions are performed in order to prevent the presence of the aforementioned factors, including rubella vaccination, blood glucose control, multivitamin administration and avoidance of teratogenic agents. The investigation of the other factors which are involved with this condition is crucial, since effective interventions in this regard would result in preventing the occurrence of CHD.
2. Objectives
This study aims to investigate the role of several potential factors associated with the occurrence of CHD.
3. Methods
This study was conducted as a cross-sectional case-control study, in Imam Khomeini Hospital Complex, Tehran, Iran. A total of 1338 known case of CHD, diagnosed by echocardiography or angiography and 1201 healthy children as controls were included in this study. Several information was obtained from the enrolled participants, including age, gender, birth weight, family history of CHD, type of CHD, parents’ consanguinity, history of previous abortion or stillbirth, maternal age, maternal past medical history, maternal drug consumption during pregnancy, and type of delivery. The patients’ origin is described in Table 1. All collected data were analyzed by SPSS version 22. The qualitative variables were expressed by number and percent. The quantitative variables were expressed by mean and standard deviation. The risk factors among cases and controls were compared using Pearson χ2. P-values below 0.05 was considered as statistically significant.
| Case, % | Control, % | Difference, % | |
|---|---|---|---|
| Tehran | 24.5 | 27.7 | 3.2 |
| Other cities | 57 | 57.1 | 0.1 |
| Center of province | 13.5 | 8.6 | 4.9 |
4. Results
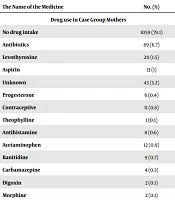
The mean birth weight in case and control group were 3 (+ 0.648) and 2.9 (+ 0.707) kilograms, respectively. The types of CHD were not significantly different between cases and controls (P < 0.05); 41% had ventricular septal defects; 32% had atrial septal defect, 7.1% had aortic stenosis, 3.3% had transposition of the great arteries, 2.9% had single ventricle and 3.3% had complex CHD. The maternal age in 983 (73.4%) cases and 960 (79.93%) controls was below the 30 years, the maternal age for other participants was above the 30 years (P-value < 0.001). the parents’ consanguinity was positive for 502 (39.24%) cases and 386 (32.19%) controls (P-value < 0.001). positive family history of CHD (among siblings) was detected in 55 (4.2%) cases and 5 (0.42%) controls (P-value < 0.001). History of previous abortion was positive in 336 (25.67%) cases and in 263 (21.92%) controls (P-value < 0.001). history of stillbirth for the mothers was positive for 63 (4.86%) cases and 48 (4.02%) controls, without any significant differences (P-value = 0.18). The drug consumption during pregnancy was positive for 182 (16.71%) cases and 112 (9.33%) controls (Table 2). Maternal history of chronic disease was found in 113 (9%) cases and 113 (11%) controls (Table 3).
| The Name of the Medicine | No. (%) |
|---|---|
| Drug use in Case Group Mothers | |
| No drug intake | 1059 (79.1) |
| Antibiotics | 89 (6.7) |
| Levothyroxine | 20 (1.5) |
| Aspirin | 13 (1) |
| Unknown | 43 (3.2) |
| Progesterone | 6 (0.4) |
| Contraceptive | 11 (0.8) |
| Theophylline | 1 (0.1) |
| Antihistamine | 8 (0.6) |
| Acetaminophen | 12 (0.9) |
| Ranitidine | 9 (0.7) |
| Carbamazepine | 4 (0.3) |
| Digoxin | 2 (0.1) |
| Morphine | 2 (0.1) |
| Phenobarbital | 2 (0.1) |
| Metoclopramide | 2 (0.1) |
| Heparin | 5 (0.4) |
| Diazepam | 1 (0.1) |
| Corticosteroids | 2 (0.1) |
| Propranolol | 2 (0.1) |
| Anti-depressant | 3 (0.2) |
| Methyl dopa | 1 (0.1) |
| Codeine | 1 (0.1) |
| Methyl Guanine | 1 (0.1) |
| Ibuprofen | 1 (0.1) |
| Omeprazole | 1 (0.1) |
| Clomiphene | 1 (0.1) |
| Drug in Mothers in the Control Group | |
| No use | 1088 (90.6) |
| Multi vitamin | 24 (2) |
| Acetaminophen | 17 (1.4) |
| Iron tablet | 17 (1.4) |
| Insulin | 19 (1.6) |
| Levothyroxine | 12 (1) |
| Antibiotics | 24 (2) |
| Disease | No. (%) |
|---|---|
| Maternal Past Medical History in the Case Group | |
| Upper respiratory tract infection | 20 (1.5) |
| Urinary tract infection | 39 (2.9) |
| Hypertension | 3 (2.5) |
| Infection of the lower genitalia | 11 (0.8) |
| Anemia | 29 (2.2) |
| Endocrine diseases | 35 (2.6) |
| Other infections | 13 (1) |
| Heart disease | 8 (0.6) |
| Gastrointestinal diseases | 4 (0.3) |
| Epilepsy | 5 (0.4) |
| No history of the diseases | 1084 (81) |
| Maternal Past Medical History in the Control Group | |
| Gestational diabetes | 35 (2.9) |
| Upper respiratory tract infection | 19 (1.6) |
| Hypertension | 32 (2.7) |
| Urinary tract infection | 21 (1.7) |
| Infection of the lower genitalia | 3 (0.2) |
| Hypothyroidism | 3 (0.2) |
| No history of the diseases | 1068 (88.9) |
5. Discussion
The results of this study indicated that the prevalence of maternal age above 30 years, positive parents’ consanguinity and maternal previous history of abortion was significantly higher among patients with CHD; furthermore, positive history of CHD among siblings of the known cases of CHD was higher than healthy ones, this calls for cardiac evaluations of all offspring of the family, in case CHD is confirmed in one of them.
Many risk factors have been identified to be associated with the occurrence of CHD, many of them are preventable by an appropriate intervention, such as anti-rubella vaccination; however, the interventions regarding the other risk factors, such as diabetes mellitus is more complicated and difficult. The primary prevention of the cardiac defects was first suggested by a clinical trial that indicated the efficacy of the folic acid to reduce the occurrence of the neural tube defects (8). There is a considerable lack of information regarding the preventable risk factors of CHD. This gap makes it difficult to run population-based strategies to decline the burden of CHD and also to educate the parents to change the lifestyle to reduce the risk of CHD.
Maternal chronic diseases, including diabetes, hypertension, anemia, connective tissue disorders, epilepsy and mood disorders, predispose fetus at a higher risk of CHDs. The management of the aforementioned disorders as much as possible, besides more frequent prenatal screening is recommended for these women (7).
Several studies have found that the increased maternal age during the pregnancy is associated with a higher occurrence of CHD (9). Chou et al. in 2016 found that infants born to mothers older than 35 years of age have 20% increased risk of CHD (7). Positive association between maternal fever in the first trimester and CHD has been indicated by Botto et al. (10); however different results to this study had been reported in other studies (11). The incidence and survival of CHD has been reported different, based on various ethnic and geographic groups. This implies the fact that both genetic and environment are associated with the occurrence of CHD (12). Several studies have indicated that history of maternal type 1 and type 2 diabetes, especially when it is poorly controlled, is strongly associated with the CHD in the offspring (12, 13). High blood glucose level leads to teratogenic effects on the fetal circulation development, especially before seventh weeks of gestation (13), additionally, Chou et al. in 2016 found that positive history of maternal mood disorder and epilepsy is associated with higher risk of CHD in offspring (7). Consuming anticonvulsants, tranquilizers and hypnotics are also importantly associated with the occurrence of CHD (14). Low birth weight and prematurity are considered as the risk factors of CHD, with unknown mechanisms (15, 16).
In case-control studies, it is evident that the correct selection of the control group has a significant effect on the outcome of the study. The population of this study was in fact a combination of different populations. For example, in the case group, about 50% of the population were referred for treatment from different regions. Parents’ consanguinity is more common in some regions, due to their culture. Therefore, there would be a higher risk of revealing latent genotypes. The positive history of CHD in siblings is an indicator of genetic factors associated with the occurrence of CHD from one side and the need for cardiac evaluations of other offspring once cardiac defects is confirmed in one kid, from the other side.
Regarding the drug consumption during pregnancy, it is noteworthy that there was no history of multivitamins and folic acid consumption in all mothers in the case group and only 2% of the mothers in the control group during pregnancy. These two multivitamins have been demonstrated to have protective effect on the occurrence of CHD, this finding indicated the necessity of prescribing multivitamins during pregnancy and also training of parents about the necessity of using multivitamins during periconceptional period.
Putting altogether, according to the findings, all factors except the history of stillbirth, maternal underlying disease and drug use during pregnancy in the case group were significantly different with the control group. It seems that the causes of CHD in Iran are most commonly related to the hereditary genetic factors; and increased maternal age, drug use, and underlying diseases in the mother, are a matter of less importance.