1. Background
Primary pulmonary arterial hypertension (PAH) or idiopathic pulmonary arterial hypertension is a rare and progressive disease with poor prognosis affecting the precapillary pulmonary arteries and is defined as a mean pulmonary artery pressure (mPAP) of higher than 25 mmHg (1). It is mostly presented with shortness of breath, grunting, cool and clammy extremities, hypotension, reduced blood oxygen level, and so on. The prevalence of PAH is 4 - 6 million individuals around the world. Annually, 140 mortalities due to PAH are reported in the USA (2). The PAH may occur at any age with an equal prevalence in both genders; however, it is higher among females than males if it is diagnosed at an older age (1: 1.7) (3).
Although the incidence of PAH is rare, its mortality rate is high if it remains untreated (4, 5). Disease progression in both children and adults with PAH is rapid (possibly faster in children). Moreover, right ventricular failure, clinical deterioration, and mortality due to untreated and pulmonary vascular resistance are reported in both children and adults with PAH (6).
Based on the National Institutes of Health Registry, the median untreated survival rate after the diagnosis of PAH is shorter in children as compared to adults (10 months versus 2.8 years) (7). The main challenges of pediatric PAH are timely detection and appropriate treatment strategies (8). There is a similar medical management algorithm for children and adults with pulmonary vascular disease (9, 10). Recently, some improvements in the prognosis of children with PAH have been reported due to the advances in the perception of the pathophysiology of the disease and its new therapies (11).
However, the observation of PAH, whether actively sought or incidentally uncovered on echocardiogram or right heart catheterization, deserves more extensive research on the etiology, perception of the pathophysiology, and appreciation of treatment options. Although the incidence of PAH is very low (i.e., about 4 to 6 per million per year worldwide), its prognosis is very poor, especially in patients with a late diagnosis.
2. Objectives
Considering the paucity of research on pediatric PAH in Iran, the present study aimed to evaluate the clinical and paraclinical findings of PAH in children in order to help with a better perception of the disease, early diagnosis, and treatment.
3. Methods
This cross-sectional study was carried out on the PAH children who were assessed in terms of PH or hospitalized at the cardiac ward January 2018 to October 2001.
3.1. Inclusion and Exclusion Criteria
The inclusion criteria were a maximum age of 18 years and a definite diagnosis of primary pulmonary hypertension (PPH). All the medical records of patients were reviewed, and those with other causes of PH, including pulmonary and hematologic problems, were excluded. All patients were examined and those presenting with alternative causes of PH, such as pulmonary or hematologic issues, were ruled out. Only PAH patients were included in this investigation.
3.2. Study Design
In this study, the data were collected from the medical records of the patients who were assessed in terms of PH or hospitalized at the cardiac ward January 2018 to October 2001. In general, 20 patients were selected using the census method. The data were extracted from medical records and recorded in a researcher-made questionnaire.
An echocardiogram (Resona 7 and HS 70) with 3 - 5 MHz Multi-frequency probe (Samsung and Mindray Company) was used to provide the images of the heart’s valves and chambers. Moreover, computed tomography angiography (Siemens Healthcare, Forchheim, Germany) was applied to produce the images of blood vessels and tissues.
The collected data included age (at the time of referral), gender, birth order, family history, parental relationship, birth weight, weight on admission, and type of delivery. Moreover, clinical signs and symptoms and mortality, were recorded in a checklist. Cardiac catheterization findings were extracted from the medical records. Chest X-ray (CXR) and electrocardiographic findings included cardiomegaly, right atrial enlargement (RAE), right ventricular hypertrophy (RVH), etc.
3.3. Statistical Analysis
In the present study, SPSS software (version 16) was used to analyze the data. Mean and standard deviation were applied for the description of the quantitative data, and the qualitative variables were explained via frequency and percentage. The independent t-test and its non-parametric test were utilized for the quantitative variables. In addition, the chi-square test was employed for the qualitative variables. Pearson or Spearman correlation coefficient was used for the assessment of the relationship between the variables considering normality. A P-value of less than 0.05 was considered statistically significant.
3.4. Ethical Considerations
The protocol of the present study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (no.: 960658; code: IR.MUMS.fm.REC.1396.712). The collected data were coded to maintain the confidentiality of the information.
4. Results
In this study, the data were collected from the medical records of 20 patients hospitalized at the cardiac ward. The mean age and birth weight of the subjects were 6.49 ± 4.82 years and 3.24 ± 0.69 kg, respectively. Table 1 illustrates the demographic data and clinical symptoms. Some patients with neurological complaints, such as seizures and syncope, were referred to a neurologist and treated with anticonvulsants.
| Variable | No. (%) |
|---|---|
| Demographic Data | |
| Age (y) | |
| 0 - 5 | 10 (50) |
| 6 - 11 | 5 (25) |
| 12 - 18 | 5 (25) |
| Gender | |
| Male | 6 (30) |
| Female | 14 (70) |
| Birth order | |
| First | 6 (30) |
| Second | 6 (30) |
| Third | 1 (5) |
| Fourth | 2 (10) |
| Fifth | 2 (10) |
| Sixth | 2 (10) |
| Seventh | 1 (5) |
| Family history | |
| Yes | 0 (0) |
| No | 20 (100) |
| Parental relationship | |
| Consanguineous | 4 (25) |
| Non - consanguineous | 12 (75) |
| Birth weight (kg) | |
| < 2.5 | 2 (11.8) |
| 2.5 - 4 | 13 (76.5) |
| > 4 | 2 (11.8) |
| Type of delivery | |
| Normal | 13 (72.2) |
| Cesarean section | 5 (27.8) |
| History of seizures | |
| Yes | 3 (20) |
| No | 12 (80) |
| Clinical Symptoms | |
| Functional dyspnea | |
| Yes | 17 (85) |
| No | 3 (15) |
| Shortness of breath | |
| Yes | 11 (55) |
| No | 9 (45) |
| Syncope | |
| Yes | 5 (25) |
| No | 15 (75) |
| Cyanosis | |
| Yes | 13 (65) |
| No | 7 (35) |
| Palpitations | |
| Yes | 6 (30) |
| No | 14 (70) |
| Heart murmurs | |
| Yes | 15 (83.3) |
| No | 3 (16.7) |
| Chest pain | |
| Yes | 6 (31.6) |
| No | 13 (68.4) |
| Edema | |
| Yes | 4 (20) |
| No | 16 (80) |
| Hepatomegaly and ascites | |
| Yes | 2 (10) |
| No | 18 (90) |
| Ascites | |
| Yes | 2 (10) |
| No | 18 (90) |
Cardiomegaly, RAE, RVH, and prominent pulmonary conus were reported in 62.5%, 75%, 75%, and 81.2% of the patients, respectively. Pulmonary vascular marking was normal in 31.2% of the subjects. In addition, pulmonary vascular marking increased and decreased in 56.2% and 12.5% of the patients, respectively.
The assessment of the relationship between age and birth order with systolic pulmonary artery pressure (PAP), diastolic PAP, and mPAP showed no correlation among these variables.
As demonstrated in Table 2, there was no relationship among the clinical findings with mPAP, tricuspid regurgitation pressure gradient, and peak systolic pressure gradient (P > 0.05). A comparison between the patients with different age groups showed that there was no significant difference between age with functional dyspnea (P = 0.17), syncope (P = 0.301), cyanosis (P = 0.53), heart murmurs (P = 0.49), chest pain (P = 0.15), edema (P = 0.08), hepatomegaly (P = 0.32), and ascites (P = 0.32) among the children with PAH. However, a significant difference was observed between palpitations and children with PAH at different age groups (P = 0.01).
| Clinical Symptoms | Pulmonary Artery Pressure | Tricuspid Regurgitation Pressure Gradient | Peak Systolic Pressure Gradient | |||
|---|---|---|---|---|---|---|
| Mean ± SD | P-Value | Mean ± SD | P-Value | Mean ± SD | P-Value | |
| Functional dyspnea | 0.83 | 0.67 | 0.93 | |||
| Yes | 66.6 ± 22.1 | 75.2 ± 24.2 | 44 ± 14 | |||
| No | 63.6 ± 24 | 81.6 ± 16.07 | 45 ± 7.07 | |||
| Shortness of breath | 0.84 | 0.16 | 0.55 | |||
| Yes | 67.1 ± 26.8 | 68.6 ± 21.1 | 42 ± 16.8 | |||
| No | 65.1 ± 15.9 | 84 ± 23.03 | 47.5 ± 5 | |||
| Syncope | 0.45 | 0.906 | 0.28 | |||
| Yes | 72.6 ± 9.8 | 77.4 ± 14.01 | 5 ± 2.4 | |||
| No | 63.8 ± 24.6 | 75.9 ± 25.8 | 40.3 ± 16.2 | |||
| Cyanosis | 0.59 | 0.65 | 0.054 | |||
| Yes | 68.5 ± 11.7 | 78.3 ± 19.6 | 52 ± 4.9 | |||
| No | 62.8 ± 31.6 | 73.1 ± 28.4 | 36.4 ± 16.6 | |||
| Palpitations | 0.057 | 0.18 | 0.509 | |||
| Yes | 80 ± 30.6 | 88 ± 29.7 | 39.6 ± 15.3 | |||
| No | 89.7 ± 13.4 | 71.8 ± 19.06 | 46.14 ± 12.9 | |||
| Heart murmurs | 0.83 | 0.16 | 0.52 | |||
| Yes | 67.9 ± 21.9 | 82.15 ± 20.3 | 45.2 ± 12.6 | |||
| No | 71.5 ± 19 | 61.6 ± 27.5 | 51.5 ± 2.1 | |||
| Chest pain | 0.208 | 0.67 | 0.93 | |||
| Yes | 75.8 ± 30.5 | 23.8 | 72.2 ± | 43.7 ± 14.5 | ||
| No | 61.4 ± 16.6 | 77.7 ± 24.2 | 44.5 ± 13.6 | |||
| Edema | 0.37 | 0.91 | 0.901 | |||
| Yes | 57.2 ± 15.3 | 77.5 ± 17.07 | 43.33 ± 20.8 | |||
| No | 68.5 ± 23.05 | 76 ± 24.7 | 44.5 ± 10.7 | |||
| Hepatomegaly | 0.36 | 0.47 | -- | |||
| Yes | 52.5 ± 24.7 | 65 ± 7.07 | 20 ± 0 | |||
| No | 67.7 ± 21.6 | 77.7 ± 23.8 | 46.8 ± 10.6 | |||
| Ascites | 0.36 | 0.47 | -- | |||
| Yes | 52.5 ± 24.7 | 20 ± 0 | 65 ± 7.07 | |||
| No | 67.7 ± 21.6 | 46.88 ± 10.6 | 77.7 ± 23.8 | |||
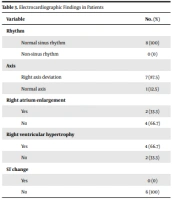
In electrocardiography heart rate was 124.12 ± 21.72. Rhythm, axis and other findings are shown in Table 3.
| Variable | No. (%) |
|---|---|
| Rhythm | |
| Normal sinus rhythm | 8 (100) |
| Non-sinus rhythm | 0 (0) |
| Axis | |
| Right axis deviation | 7 (87.5) |
| Normal axis | 1 (12.5) |
| Right atrium enlargement | |
| Yes | 2 (33.3) |
| No | 4 (66.7) |
| Right ventricular hypertrophy | |
| Yes | 4 (66.7) |
| No | 2 (33.3) |
| ST change | |
| Yes | 0 (0) |
| No | 6 (100) |
In total, 56.3% of the patients expired. The frequency of non-survived patients (50%) was higher in the age group of 0 - 5 years. In addition, two quarters of the non-survived patients were within the age ranges of 6 - 11 and 12 - 18 years (each, 25%). Table 3 presents the relationship between the survival rate and clinical findings.
5. Discussion
In summary, the results of the present study showed that the occurrence of PAH was higher among the females. The majority of the patients had no family history and their parents were non-consanguineous. Most of the subjects had no history of seizures, syncope, chest pain, edema, and ascites. Moreover, functional dyspnea, shortness of breath, cyanosis, palpitations, and heart murmurs were reported in the majority of the participants. Some patients with neurological complaints, such as seizure and syncope, were referred to a neurologist and treated with anticonvulsants.
Similarly, a higher frequency of females among children with PAH is reported in other studies (11-13). Possibly, the presence of gender differences in the plasmin and thrombin activation system in PAH leading to an antifibrinolytic/prothrombotic state can explain the higher incidence rates among females (14). In general, the incidence rate of acute lung disease in neonates is estimated to be 3%, which is associated with decreasing gestational age and birth weight (15). Based on a 14-year epidemiological study, the transient forms of PAH were reported in 80% of all children, and progressive PAH accounted for 5% of all patients (16).
Preterm birth is reported as the main risk factor for PAH among children and young adults (15, 17). However, in the current study, majority of patients were term neonates. Having said that, neonatal respiratory disease and mortality rates have decreased in the post-surfactant era due to improvements in pre and postnatal care methods (18).
The rate of PH in premature births is estimated within the range of 14 - 21.8% in other studies (19, 20), which is approximately half of that reported by Naumburg et al. (15, 17). The reason could be the increasing survival rates after premature births, as shown in another similar study (21). The incidence of PH in preterm neonates may be due to impaired vascular growth resulting in a limited vascular surface (22).
Dyspnea is commonly observed in more than 90% of children with PAH (23). Shortness of breath, syncope, cyanosis, and edema were introduced as the most common symptoms of PAH in a study by Barst et al. (24) and Charalampopoulos et al. (25).
A clinical picture of pediatric PAH includes vasoconstriction in skin and kidney leading to cyanosis and acute renal shutdown (26). Cyanosis is common in children with PH and is frequently observed due to right-to-left shunting through an anatomic defect in the presence of PH (27). Syncope occurs most often in children with PAH without systemic-to-pulmonary or fully repaired shunts, which is commonly reported in the younger patients (19). In the current study, some patients with neurological complaints, such as seizure and syncope, were under the supervision of neurologists and were treated with anticonvulsants, before referring to pediatric cardiologist.
The most common CXR findings in our study population were prominent pulmonary conus, RAE and RVH. This result is in line with the findings of a study by Dixit and Alva (8). The mean age of PAH children evaluated in the current study was higher than that reported for the aforementioned study (6.5 years and 5.6 months).
The rate of cardiomegaly in the study by Dixit and Alva was nearly similar to the present study; however, the frequency of prominent pulmonary conus was higher in the current study (81% and 62%) (8). Nevertheless, based on the results of another study carried out by Kovacs et al. (28), prominent pulmonary conus was reported as 43% in PAH patients. The variations may be due to differences in demographic information or other confounding variables.
Based on the electrocardiogram (ECG) findings of the study carried out by Kovacs et al. (28) right axis deviation (RAD) was observed in 49% of patients. An abnormal ECG is more likely in severe PH rather than mild PH. The ECG has insufficient sensitivity for RVH (28). Moreover, RAD was reported in 87.5% of the patients in the current study. Based on a study performed by Galie et al., RAD is strongly correlated with the presence of PH (29). Santoso et al. analyzed the ECG of 120 subjects diagnosed with and without PH and showed that RAD is an independent predictor of PH in patients with cardiac problems (30).
In the current study, a higher mortality rate was observed among children of a younger age. Moreover, the 5-year survival rates of patients with childhood-onset PAH were estimated within the range of 71.9 - 75% in previous studies (31-33).
It is shown that there is a correlation between the maintenance of vasoreactivity and survival rate in patients with PAH (31, 34). Repeated cardiac catheterization in pediatric PAH can help in the early diagnosis of the disease, assessment of treatment effect, and prediction of prognosis. However, it should be performed at modern centers for the management of critical complications, such as the PH crisis, requiring extracorporeal membrane oxygenation (35).
5.1. Conclusions
The findings of this study suggest that there is a wide range of iPAH clinical and paraclinical signs, none of which is specific. The findings also suggest that iPAH should be considered as a possible diagnosis in various clinical manifestations, including respiratory distress, hepatomegaly and ascites and even neurological symptoms. Since there seems to be no specific symptoms therefore a set of history, physical examination, laboratory findings, and clinical suspicion should be taken into consideration.
5.2. Limitations and Weaknesses
Due to the retrospective nature of this study, the obtained results cannot be generalized to other populations. Being single-center is another important limitation of the present study. Also, testing cardiac biomarkers in IPAH, which could be valuable, was not performed in this study.