1. Background
Congenital heart disease (CHD) is the most common life-threatening neonatal cardiovascular disease with a high prevalence up to 12 per 1000 live births. It is considered as a fundamental malformation of cardiac structure or great thoracic arteries and is often missed during routine diagnosis (1-5). Nearly 50% of all newborns suffer from major CHDs, which necessitate corrective or palliative surgery in the first years of their lives (6). Stillbirth is associated with CHD in over half of the cases, and being accompanied by extra-cardiac anomalies, the risk of morbidity and mortality ramps up even further (7). Prenatal diagnostics, which has been utilized for more than 30 years, allows us to detect a heart lesion early and make an appropriate delivery decision (8). Detection of most cardiac structural anomalies should be carried out around 18 - 20 weeks of gestational age. Prenatal diagnoses could be achieved by ultrasonography (US) or fetal echocardiography (FE). Studies showed obstetrics ultrasonography was not able to diagnose CHD in more than 15000 low-risk pregnancies and structured cardiac anomalies could be missed by ultrasonography due to cardiac complicated anatomy and its continuous motion (8-10). As a result, fetal echocardiography is seen to be a superior option for detecting prenatal CHD due to its high sensitivity and specificity. Fetal echocardiography has increased the number of images available in the four-chamber echocardiogram and improved the accuracy of CHD diagnosis. It is obvious that the consequences of misdiagnosis comprises of what may happen to the fetus as an individual, its psychological and financial impact on anxious parents, its influence on medical care expenses, and how it complicates management and treatment (8). Although accurate and detailed diagnosis is important, determining the plan for the fetus as a patient, his mother, and also the treatments require more emphasize on the FE which is performed by a person or a team completely familiar with cardiac disease and its management during the perinatal period and after the birth, as well.
2. Objectives
To the best of our knowledge, this is the first study in Iran to examine and compare ultrasonography and echocardiogram data in order to determine which approach is more effective for accurate prenatal diagnosis of CHD and related variables (11). Based on the observations it is recommended that detailed FE is going to be a part of routine fetal screening soon, but until then we should follow the current guideline. Although, accurate diagnosis is important; the management of the disease is a more important issue that should preferably be attended by a team including a perinatologist, radiologist, and pediatric cardiologist.
3. Methods
This was a retrospective, cross-sectional, non-randomized study, planned for seven years from 2012 to 2019. All pregnant women in 1st trimester of gestation for fetal cardiac examination were referred and presented to Imam Reza hospital, a university tertiary heart center in Mashhad, Iran. Although those who presented to our center later were examined at their first presentation. The examination was conducted by a single skilled pediatric cardiologist to avoid bias, one who is the director of the congenital and children’s cardiac unit, as a specialized academic referral children heart-center. Detailed fetal echocardiography was performed on GE Vivid 7 color Doppler and Mindray Resona 7 color Doppler using a 5 - 7 MHz convex probe. Also, Mothers were subjected to routine obstetrics ultrasounds which were performed by an expert radiologist in anomaly scan form. All the reports were gathered and compared to each other. The diagnostic reports are categorized into major and minor groups. Major cardiac disease refers to cases that the fetus has an important cardiac abnormality that needs to be followed up and it is very effective in determining the plan for the management of delivery and treatment during both prenatal and postnatal periods. Major cases included septal defects, right and left side obstructive lesions, great vessels abnormalities, complex CHD, CMP, myocarditis, masses, and arrhythmias. Other less important cardiac cases are categorized into minor group.
Data were analyzed by the statistical package for the social sciences (SPSS) version 18 (SPSS Inc. PASW Statistics for Windows, version 18.0. Chicago: SPSS Inc.). Continuous data were expressed as mean ± standard deviation (SD). The categorical variables and related frequencies were shown in percentage. The accuracy of the ultrasound in diagnosing CHD was assessed by chi-square and Fisher’s exact tests The agreement between two methods in CHD detection was evaluated by using Cohen’s kappa coefficient (K > 0.4, P < 0.05 as significant cut-off).
The sensitivity and specialty, accuracy, positive and negative predictive values were determined by comparing ultrasound findings with FE examination.
The study was based on a prenatal CHD examination project which was approved by the Institutional Ethics Committee of Mashhad University of Medical Sciences. (Ethical code: IR.MUMS.fm.REC.1394.638)
4. Results
A total of 312 fetal echocardiograms were carried out by our single expert pediatric cardiologist presented to our fetal cardiac tertiary center. The mean age of mothers was 31.5 ± 5.14 (19 - 45) years and the mean gestational age on presenting examination day was 24.4 ± 6.09 (12 - 38) weeks. Mothers were mainly referred to gynecologists (75%) with the most referral reason and the abnormality of ultrasonography reports. Out of 312 studied fetuses, 219 (70.19%) were normal and 57 (18.26 %) were abnormal specifically in structure as CHD. The remaining US reports showed increased NT (2.8%), arrhythmias (4.16%), extra-cardiac abnormality (2.56%), hydrops, effusion and CHF (1.92%). The most cardiac abnormality detected in 16-19 weeks of gestational age by US (35%); which was the same as FE.
Of the 312 performed fetal echocardiography, 194 (62.1%) cases were normal, 56 and 62 cases were major and minor cardiac abnormalities, respectively. FE and US both have an agreement in finding 52 normal cases; whereas 33 cases were reported normal by the US and 14 cases were detected abnormal by FE. US considered 21 major and 36 minor abnormal cardiac which only 34 cases were confirmed by FE, of which, 82.35% were minor defects. In general, the two methods were in agreement with the content of 11.53% of the cardiac abnormalities diagnosis.
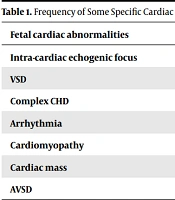
US underdiagnosed 35 major, mostly septal defects (45%) and 26 minor structural cases. On the other hand, US overdiagnosed 10 minor and 7 major cases (septal defects: 6 VSD, 1 AVSD). The details are as follows: Intra-cardiac echogenic focus was found as the most common cardiac abnormality in our FE examination 37 (12.17%); whereas US reported only 18 of these cases (7.05%), conversely US overdiagnosed 10 other cases more. Both methods detected VSD as the most common CHD, FE detected 5.12% but US 2.24%. US overdiagnosed 71.4 % of VSD detected cases and FE could not confirm those either, 87.5 % of VSD cases were only diagnosed by FE which the US was not able to detect (kappa = 0.13). The US could not detect 11 out of 13 cases of complex CHDs (84.61%) but FE was completely potent in detecting the complex CHD presence (kappa = 0.3). In addition to the structural anomalies, FE found arrhythmia in 37 (11.8 %) of cases; but the US detected 13 (4.16%), of which, only 6 cases were confirmed by FE. One out of two cases of CMP was detected by US (kappa = 0.665, P < 0.001). On searching AVSD in fetuses, the methods were in agreement up to 98% in reporting normality. Nevertheless, about one percent of detected AVSD was not even mentioned by US. Also, 0.64 % of cases were overdiagnosed by US as AVSD which means FE did not confirm (kappa = -0.01). Only one cardiac mass was diagnosed by US, out of four cases that were seen by using FE (kappa = 0.31, P < 0.001) (Table 1).
| Fetal Cardiac Abnormalities | US Detection (%) | FE Detection (%) |
|---|---|---|
| Intra-cardiac echogenic focus | 7.05 | 12.17 |
| VSD | 2.24 | 5.12 |
| Complex CHD | 0.66 | 4.13 |
| Arrhythmia | 4.16 | 11.8 |
| Cardiomyopathy | 0.33 | 0.66 |
| Cardiac mass | 0.33 | 1.32 |
| AVSD | 0.64 | 1 |
US reports mentioned increased NT (nuchal translucency) in nine fetuses and FE confirmed CHD in only two of them; although US did not report it directly. It reported five fetuses suffered from hydrops, effusion, and CHF; but fetal echocardiography confirmed only one of the detected cardiac abnormalities (P < 0.001, Kappa = 0.23).
The sensitivity, specificity, and accuracy of the prenatal diagnostic by US compared to FE were estimated at 62%, 74.2%, and 68.7% respectively. Also, the positive and negative predictive values calculated 66.1% and 70.27%.
5. Discussion
Congenital heart disease as the most common congenital malformation is necessary to be detected properly before birth. Accurate prenatal diagnosis improves the fetus and neonate survival, especially for those who needed to have prostaglandin injected and to acquire invasive intervention or immediate surgery. Prenatal diagnosis of CHD not only distinguishes cardiac defects but also finds associated abnormalities (12-14).
Ultrasound made fundamental changes in prenatal diagnosis but detection of major fetal CHDs has not improved as much as other anomalies. Many studies showed cardiac anomalies are regularly missed during sonographic scans. The detailed complexity of cardiac structural anatomy and its dynamic nature makes it hard to evaluate. FE modality has expanded remarkably since 1972 and now represents a highly specialized achievement and a sensitive technology in prenatal cardiac care (both more than 90%). Also, the skilled hands optimize the effectiveness of cardiac examination details (15).
In this study, we compare US reports to FE in the prenatal diagnosis of CHD in a group of 312 pregnant women, and we examine the results to see which one can identify CHD more accurately. We hypothesized that applying fetal echocardiographs in routine scans improves prenatal detection rates and reduces missing cases. We should be aware of some significant issues which required precise diagnoses such as the accuracy of diagnostic tools, the effectiveness in the prenatal outcome, suitable prenatal counseling, optimal management, and superlative treatment.
Prenatal US screening of congenital malformation is suggested and performed in the first trimester (11 - 13 wks. of GA) and second trimester (18 - 22); the same time seems appropriate for performing FE. As most studies showed the most referral cause was from a gynecologist which was consistent with our findings (16, 17).
Both US and FE agreed that increased echogenicity is the most prevalent found abnormality which is almost expected. As late studies predicted, VSD is considered the most frequent CHD; however, US missed some cases (17). Due to the detailed anatomy of complex CHD and its specialized expert requirement, it is often missed to be detected by US but not FE. Furthermore, there were several overdiagnosed VSDs, which incurred significant financial and psychological costs for the healthcare system as well as the parents. Diagnosis of other CHD cases and also arrhythmias proved US did not perform practical enough in comparison with FE. Strikingly, US mostly diagnosed minor cardiac abnormal cases but is not capable of detecting the major ones affecting CHD prognosis.
In general, there was a connection between the results of US and FE, but there was no substantial agreement in definitive diagnosis, which has become an issue, resulting in delayed proper diagnosis, misdiagnosis, and treatment delays. In some developed countries, national screening programs of congenital structural cardiac anomalies are routinely performed by professionals trained for FE. Thus, higher accuracy in the presence of pediatric cardiology is successfully achieved and the national program ensures that no cases even low-risk ones are not missed (18). Due to the importance of the issue and therapeutic complications the high mortality of cardiac diseases and the resulting socioeconomic and psychological effects, FE has progressed even in developing countries. And it appears that using FE by trained individuals at least once for all pregnant women is advantageous, and it may lower the expense of different misdiagnoses, including fetal mortality, and apply the cost-benefit treatment. Unfortunately, in developing countries like ours, limitation of FE availability in all centers and high cost prohibited it to some extent (19). Therefore, it is still better to follow the updated local guidelines in each area especially in form of a team including a pediatric cardiologist and a perinatologist in addition to a radiologist. Another issue that needs to be considered is in most US cases there was no advice or at least an appropriate one, only abortion advice was sometimes mentioned.
5.1. Conclusions
Our study confirmed that some proportion of CHDs were notably missed in US evaluation. On the other hand, FE was very efficient and potent for accurate diagnosis. Although there was a valid correlation between the results, there was no significant agreement of accurate diagnosis and its resulting advice. About minor CHDs it is partly acceptable; but about major diseases that needed to be followed up and interfered with, the results were not satisfactory. Also, the accurate diagnosis of arrhythmia was acceptable using echocardiography in comparision with US. Although fetal echocardiography is still the best method for prenatal diagnosis and studies as ours strongly encourage to perform fetal echocardiography for all pregnant women at least once; according to updated guidelines still is not acceptable for applying in all regions perhaps due to resource limitations and cost-benefit issues.
5.2. Strength and Limitations
FE evaluations were performed by a skilled pediatric cardiologist and gathered US reports were all performed by an expert in the form of an anomaly scan. Also, the sample size was sufficient. It might be preferable to follow the pregnancies –the postnatal period– and continue this research. Also, multicenter surveys are required to move towards a more comprehensive view.