1. Introduction
Heart failure (HF) remains a rising global epidemic with an estimated prevalence of > 37.7 million individuals globally. HF is associated with increased morbidity and mortality, and confers a substantial burden to the health-care system (1). Comorbidities include sarcopenia, renal impairment, cachexia, anemia, chronic pulmonary disease, and diabetes mellitus have been proven to be major predictors of prognosis and contribute to heart failure patients' poor quality of life (2, 3). Nutritional status and HF have strong associations (4). Individuals NYHA functional classes II and III reported a lower intake of macronutrients and micronutrients (5). Inadequate nutritional status has been linked to poor outcomes in HF patients (6, 7). Restricting protein intake may be harmful to patients with nutrition-related risk (8). Regarding the importance of good nutrition, especially adequate protein and energy intake, several trials have been conducted to demonstrate the benefits of amino acids supplementation in patients with HF. Although they have shown an improvement of exercise tolerance and, in some cases, left ventricular function, they have many limitations, namely small sample size, differences in patients’ characteristics and nutritional supplementations, and lack of data regarding outcomes (9). Furthermore, amino acid supplementation may lead to enhanced catabolic flux and exacerbate pathological remodeling and dysfunction in the post-MI hearts (10). The HF guidelines have not recommended a specific amount of protein intake (7). Generally, high protein intake is recommended for sarcopenic and malnourished patients (11, 12). On the other hand, protein restriction is recommended in patients with renal damage (13). Nonetheless, one study demonstrated that higher protein intake did not have a major impact on kidney function decline among elderly men and women (14). We found no clinical trial considering protein intake in HF patients with renal insufficiency. This study will make a meaningful difference in our knowledge about protein requirement of increasing number of HF patients.
2. Objectives
Primary Objectives: To evaluate the changes from baseline in muscle mass at 6 months of treatment for both diets
Secondary Objectives: To evaluate the changes in quality of life using Iranian heart failure quality of life questionnaire (IHF-QOL)
-To evaluate changes from baseline in Glomerular filtration rate (GFR) at 6 months of treatment for both diets
-To evaluate appetite using Simplified Nutritional Appetite Questionnaire (SNAQ)
-To evaluate changes from baseline in gait speed (timed 4-metre walk), sit-to-stand time (timed test of five chair rises), and standing balance (side-by-side stand, tandem and semi-tandem positions) at 6 months of treatment for both diets
-To evaluate changes from baseline in handgrip strength using handgrip dynamometer at 6 months of treatment for both diets
-To assess re-hospitalization frequency at 6 months of treatment for both diets
-To assess mortality rate at one year of treatment for both diets
As the primary outcome, we will look at the effect of protein consumption on muscle mass. Re-hospitalization, quality of life, physical performance, renal function, appetite and mortality are second endpoints.
3. Methods
3.1. Study Design
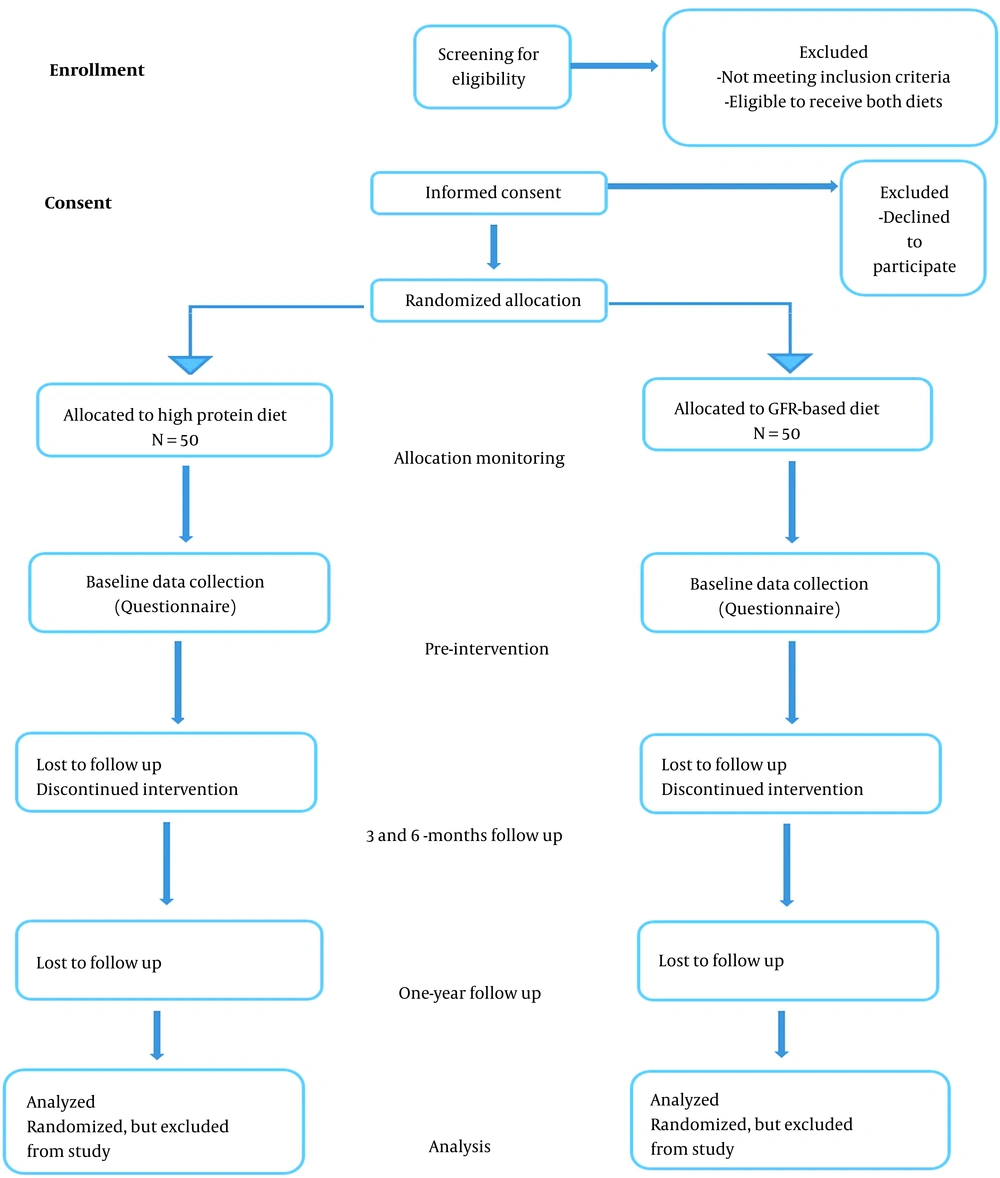
A randomized, parallel-arm, controlled pilot study is designed. Sample size is considered 50 participants per treatment arm (Figure 1). After baseline assessments, balanced permuted block method will be used for randomization. Concealment of the randomization sequence will be performed by the RCT center of Rajaie Cardiovascular, Medical and Research Center. Intervention will be according to the randomization sequence.
Following examinations will be performed for each patient at the beginning and after six months.
•To assess the efficacy of protein intake on the percentage of muscle mass that will be measured by Dual-Energy X-Ray Absorptiometry (DEXA).
•To assess efficacy of protein intake on physical performance that will be measured by Short Physical Performance Battery (SPPB).
•To determine the safety of protein intake on GFR rate that will be measured by gamma counter.
Following examinations will be performed for each patient at the beginning and after one year.
•To compare the number of readmission between high protein diet and GFR based protein diet groups.
•To compare the percentage of mortality rate between high protein diet and GFR based protein diet groups.
3.2. Subject Selection
Non-obese adult HF patients with a diagnosis of advanced heart failure (stage D) according to the American and European heart failure guidelines in New York Heart Association (NYHA) class of III (3), who are under observation and management of heart failure in transplantation department of Rajaie Cardiovascular Medical and Research Center will be included in the study. Other criteria for participant selection will be glomerular filtration rate (GFR) 30 - 90, hand grip strength less than normal for age (15), and muscle mass >2 SDs below mean in individuals aged 18 - 39 years in the NHANES III cohort (2).
Subjects with respiratory failure, ventilator dependent, sepsis, dialysis treatment, open abdominal surgery within 6 weeks prior to enrolment, and disorders or conditions that might change the calorie and macronutrients requirement will be excluded from the study.
3.3. Subject Recruitment
3.3.1. Randomization
A randomized, parallel-arm, controlled preliminary study is designed. After baseline assessments, patients who are eligible to receive both diets and sign the informed consent will be recruited for the study. Balanced permuted block method will be used for randomization. Sample number is 4 in each block. Randomization will be performed by a software in RHC which generates sequences of random digits. Allocation will be equal between treatment arms which will be performed by one of the staff via software. Concealment of the randomization sequence will be performed by the RCT center of Rajaie Cardiovascular, Medical and Research Center. Participants and care providers are not blinded to the process.
3.4. Trial Intervention
3.4.1. General Information
The individualized diet including 6 - 8 small meals will be formulated by a dietitian. For each participant, weighed food records of 3 consecutive days will be analyzed for the estimation of calorie and macronutrient intake and dietary preferences. Nutritionists-4 software (16) will be used for calorie and macronutrient intake analysis.
Study participants will be allocated to receive either a high protein diet as recommended to preserve muscle mass (1.2 - 1.5 g/kg IBW) (17) or GFR based protein intake for 24 weeks (18). As required, oral nutrition support (ONS) will be considered to meet calorie and protein needs. In subjects with lower intake of protein, polyunsaturated fat will be substituted. Nutritional interaction of prescribed drugs will be considered in diet planning for each subject individually. All patients will be educated for salt and fluid intake. Diets were tailored to each patient's eating habits and preferences in order to ensure good compliance. Patients have also received two 30-minute small group sessions and two 15 - 20-minute private counseling sessions with a dietitian through video conferences.
Before initiating intervention, individuals will be assessed for anemia and electrolytes status. Any insufficiency or imbalance will be corrected by drug therapy or supplementation.
Follow up duration will be at month 3 and 6 for outcomes other than mortality. Data for mortality will be collected in one year from the beginning of the intervention. Patients will be reminded via text massage the day before each follow-up appointment. If someone does not show up for a visit, a member of the RCT staff will call her/him, a designated friend, or a family member. A heart failure specialist will supervise the whole process of the study.
3.4.2. Measurements
Baseline data will be gathered on demographic and clinical characteristics, medical history, treatments and medications. All laboratory, anthropometric and questionnaire based assessments will be performed at baseline and 3 and 6 months’ follow-up.
3.4.3. Laboratory Measures
Routine laboratory tests for heart failure patients at RHC, including complete blood count (CBC), electrolytes status, lactate, blood urea nitrogen (BUN), creatinine, uric acid, ferritin, B-type natriuretic peptide (BNP) or ProBNP, HbA1C, thyroid function, albumin and urine analysis, will be measured using standard laboratory methods.
3.4.4. Anthropometric Measures, and Sarcopenia
Anthropometric measurements will be weight (digital weight scale, Seca), height (Portable Stadiometer- Seca) and hip and waist circumference (Ergonomic Circumference Measuring Tape, Seca). For sarcopenia, body composition, muscle mass, hand grip strength and short physical performance battery (SPPB) (19) will be assessed. Total body composition and percentage of body fat as well as fat free mass for arms, legs and trunk as well as abdominal obesity will be assessed using dual-energy X-Ray absorptiometry ((DEXA), Hologic). Handgrip strength will be assessed by hand dynamometer (JAMAR® Hand Dynamometer). SPPB including gait speed (timed 4-metre walk), sit-to-stand time (timed test of five chair rises), and standing balance (side-by-side stand, tandem and semi-tandem positions) (20) will measure physical performance.
3.4.5. Kidney Function
To assess kidney function at baseline and the end of the study, urine analysis, BUN and serum creatinine assessment (colorimetric assay) as well as glomerular filtration rate (GFR) estimation (WIZARD® Automatic Gamma Counter) (21) will be performed.
3.4.6. Questionnaire-based Assessment
Appetite assessment will be performed using Simplified Nutritional Appetite Questionnaire (SNAQ) (22). This 4-item instrument has been validated in older adults and its psychometric properties evaluated in HF patients (23). This self-assessment tool measures appetite, satiety, taste of food and number of meals per day. Total score ranges from 4 to 20 and cutoff value equal or less than 14 is considered malnutrition. For quality of life, Iranian heart failure quality of life questionnaire will be used (24). A qualified staff member will complete this questionnaire. Assessment will be performed at baseline and end of the follow-up.
3.4.7. Laboratory Markers
Laboratory markers will be measured using standard methods. CBC, electrolytes, albumin, lactate, uric acid, BUN and creatinine using colorimetric assay. BNP or ProBNP, thyroid hormones and ferritin, using ELISA, and HbA1C using HPLC, (chemiluminescent immunoassay).
3.5. Data Handling and Record Keeping
Data will be collected and recorded by trained staffs in two forms of hard copy documents and electronic program which is developed in RHC. Data quality will be assessed by double entry in a software.
3.6. Statistics
3.6.1. Sample Size
Because effect size could not be confidently predicted in the absence of relevant data, sample size was not determined statistically using standard power analysis methods. This is a preliminary, exploratory study and due to resource limitation, 100 participants (in two equal groups) will be recruited.
3.6.2. Statistical Methods
Data will be described as mean ± SD for normally distributed interval, median (inter-quartile range) for skewed interval and frequency (percentage) for categorical variables. Comparisons between two groups for the interval study endpoints and their changes will be performed via independent sample t-test and repeated measure analysis of variance models and their non-parametric equivalents, including: Mann Whitney U and Friedman’s tests. Binary endpoints will be compared using Pearson’s chi square test. P value < 0.05 will be considered statistically significant.
3.6.3. Statistical Software
Data handling will be performed using MS Excel software. Statistical analysis will be conducted via IBM SPSS Statistics 22 for Windows (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. NY, USA).
3.7. Ethics Committee Approval
The study protocol and informed consent document have been approved by the ethics committee of Rajaie Cardiovascular Medical and Research Center (ethics code: IR.IUMS.FMD.REC.1399.660). The study protocol has also been registered and approved by clinicaltrial.gov with a code number of NCT03213821. For all study participants the purpose and procedures of the study will be explained and a written informed consent will be provided before entering the study.
4. Discussion
There is a lack of evidence about the effect of high protein diet in patients with advanced HF and uremia. However, in isolated uremia (without concomitant HF) high protein diet does not seem to worsen GFR (14). In addition, some studies showed the therapeutic effects of protein/amino acid supplementation on improving heart failure, symptoms and quality of life in HF patients without uremia (25, 26). Our hypothesis is that in individuals with advanced HF, pre-existing renal impairment (a prevalent comorbidity) affects the therapeutic benefits of a high-protein diet.