1. Evidence Acquisition
1.1. Ventricular Assist Devices
Heart failure patients are increasingly being treated with ventricular assist device (VAD) therapy either as bridge-to-transplant (until a suitable heart donor is available), bridge-to-recovery, or as destination therapy (1, 2). VADs are mechanical pumps that augment or replace the function of a damaged ventricle to restore normal hemodynamics to the circulatory system, especially to maintain blood flow to vital organs (3). Since the inception of the artificial-heart program at U.S. national institutes of health (NIH) in 1964, various circulatory-support devices have been developed for short-term use in patients with end-stage heart failure (4, 5). The U.S. food and drug administration (FDA) first approved pneumatically driven left ventricular assist device (LVAD) for this purpose in 1998. These devices have predominantly found utility for two groups of patients (6).
The first group of patients require LVADs to pump blood and allow the heart to rest and recover its function. The second group consists of patients with myocardial infarction, acute myocarditis or end-stage heart disease who are not expected to recover adequate cardiac function and who require mechanical support as a bridge to transplantation (3). Currently developed VADs include extracorporeal membrane oxygenation, ventricular and biventricular extracorporeal and implantable continuous and pulsatile devices, and total artificial hearts (7).
VADs were traditionally being used to augment the function of the left or right side of the heart by displacing blood from one ventricle downstream to the patient’s vasculature. For example, an axial flow pump may be connected between the left ventricle (LV) apex and the aortic arch of the patient. The pump moves blood from the LV to aortic arch where it flows normally through the patient’s arterial system. Short-term use of these devices in patients awaiting transplantation can maintain hemodynamics, improve vital organ function and exercise tolerance, allow patients to be discharged from the hospital, and provides them with a reasonable quality of life and a relatively low incidence of major adverse events (8-15). Table 1 is showing a summary of some types of ventricular assist device.
| Device | Method | Company/Group | Regulatory Approval |
|---|---|---|---|
| Novacor | Implantable; Pulsatile | World Heart | CE, FDA, Japan |
| DeBakey | Implantable; Axial flow | MicroMed | CE, FDA |
| VentrAssist | Implantable; Centrifugal, maglev | Ventracor/Thoratec | CE, Australia |
| Jarvik | Implantable; Axial | Jarvik Heart | CE, partial FDA approval |
| Heartmate II | Implantable; Axial | Thoratec | CE, FDA (BTT, DT) |
| Heartmate III | Implantable; Centrifugal, maglev | Thoratec | Not yet approved |
| Incor | Implantable; Axial | Berlin Heart | CE |
| Excor | Paracorporeal; Pulsatile | Berlin Heart | CE, FDA (for pediatric) |
| Impella 2.5/5.0 | Percutaneous; Micro-axial | Abiomed | CE, FDA |
| MITIHeart | Implantable; Centrifugal | MiTiHeart | Not yet approved |
| HVADa | Implantable; Centrifugal, passive magnetic suspension and hydrodynamically bearing | HeartWare | CE, FDA |
| DuraHeart | Implantable; Centrifugal, maglev | Terumo | CE |
| CorAide | Implantable; Centrifugal, hydrodynamically levitated | Cleveland Clinic | Not yet approved |
| Levacor | Centrifugal | World Heart | Not yet approved |
| MiFlowa | Implantable; Mixed-flow, maglev | World Heart | Not yet approved |
Abbreviations: CE, Conformite Europeenne, meaning “European Conformity; FDA, Food and drug administration
aMinimally invasive implantation.
1.2. Complications with VADs
VADs expose a patient to potentially serious complications. The main cause of complications is direct contact of the patient’s circulation to artificial surfaces as well as the invasive surgical procedure. Hemorrhage is the most common complication associated with placement of LVAD (3). Excessive perioperative bleeding occurs between 20% and 50% of the time; however, this rate decreases as the experience with device implantation grows (16, 17). About 50% of patients require reoperation for bleeding, while death due to bleeding was reported to be in the range of 0 to 15% (17, 18). Infection is another serious complication and the primary cause of death in long-term LVAD patients. The mortality is up to 70% of LVAD recipients (3, 19). Failure of the LVAD was the second most frequent cause of death in the device group, which can occur in multiple parts of a device or in the controller (11).
By the improvement of technology, mechanical pump failure was decreased (0.03% in the 1st generation to 0% in the 2nd and 3rd generation of LVAD) (20).
Right heart failure (RHF) is also a common complication associated with VADs, occurring in nearly 40% of recipients (21). Right heart failure is also associated with high transfusion rate and increased rate of end-organ failure. The number of days in the intensive care unit and the mortality rate are also increased in RHF patients (22). Thromboembolism is an important complication that occurs in 20% of patients receiving a left or right VAD (15). The main cause of thromboembolism is the contact of device surface to blood and it depends on many factors including device profile, patient condition and anticoagulant regimen. It is due to cerebrovascular and peripheral embolization (23). Other less common complications are ventricular arrhythmias, stroke, neurological and psychological dysfunction, hemolysis and other organ dysfunctions (Table 2) (3, 19).
| Usual | Unusual |
|---|---|
| Bleeding (3, 16-18) | Hemolysis (24) |
| Infection (3, 19) | Heparin induced thrombocytopenia (25) |
| Right sided heart failure (22) | Diaphragmatic hernia (26) |
| Thromboembolism (15) | Subacute Gastric perforation (27) |
| Device malfunction (17) | Postoperative hyperbilirubinemia (28) |
| Neurological dysfunction (19) | Partial aortic valve fusion (29) |
| Organ dysfunction (19) | Interventricular septal defect (30) |
1.3. External Cardiac Compression Devices
In contrast to VADs, biventricular and univentricular ECCDs assist the failing heart by compressing cardiac and aortic tissues, thereby avoiding direct contact of blood with artificial surface. ECCDs can be further divided into two groups depending on the way they are powered. Devices in the first group are powered by an extracorporeal source such as pneumatic or electrical drives. Devices in the second group are powered by energy sources within the patient’s body like Badylak or Chiu Devise which are described below. Table 3 shows a summary of recently developed ECCDs.
| Device | Mechanism | DS | PS | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Rubin | Cup like compression | Patent | EP (EC) | NBC, UES | CEC (because of its size and immobility) |
| Myovad | Cup like compression | Clinical trial | EP (EC) | NBC, UES, Quickly and simple installing | CEC |
| Heilman | Finger like compression | Patent | EP (EC, BP) | NBC, arrhythmia control | CEC, CD |
| Wilk | Finger like compression Decrease ventricle volume | Patent | EP (EC) | UES | CD, Invasive and contact Blood in close off model |
| Shahinpoor | Finger like compression | Patent and prototype | EP (EC) | NBC, Arrhythmia control, New technology | CD |
| Sunshine Heart C-Pulse Cuff | Ascending aorta compressions | Clinical trials | EP (BP) | NBC, Small and simple device | Low pumping power |
| Chandrasekar | Finger like compression | Patent | EP (BP) | NBC, install from abdomen | Many movement parts |
| Badylak | Transplanted muscle compressing sac pulsatile | Patent | IP | NBC, using muscle power | Surgery includes expose and preserve of muscle, vascular or nerve are difficult, No UES |
| Chiu | Cup like compression | Patent | IP | NBC, using muscle power | Coordination between heart pulse and muscle contraction is difficult and more contraction is discomfort, No UES |
| Campbell | Pumping blood | Patent | IP | NBC, using muscle power | Surgery includes expose and preserve of muscle, vascular or nerve are difficult, No UES |
| Acorn CorCap | Passive support of damaged and recovering ventricles | Approved in Europe | No need for power | NBC, fast and minimally invasive installation, support cardiac chambers | No pumping blood |
Abbreviations: BP, Battery package; CD, Complicated device; CEC, Completed external console; DS, development state; EC, External console; EP, External power; IP, Intracorporeal power; NBC, No blood contact; PS, Power source; UES, usable for emergency situation.
2. Extracorporeal Powered ECCDs
Recent ECCDs developments involve some form of flexible bladder within a support structure such that expansion of the bladder presses on the ventricular tissue to facilitate expulsion of blood out of the ventricles. For example, Rubin et al designed a bladder that is inserted via an incision in the wall of the upper abdomen (31). This bladder assembly covers a significant portion of the outer surface of the ventricles. The ECCD is attached to a transcutaneous gas tube that alternates filling and depleting the bladder, compressing the left ventricle to simulate systole by expelling blood into the aorta and then filling again.
Another ECCD with a sac-like structure, the MYO-VAD, employs a direct mechanical ventricular actuation (DMVA) and is also a non-blood-contacting, biventricular assist device. The device is contoured to fit over the heart and attaches to the ventricular myocardium by an atraumatic vacuum seal. A pneumatic drive is then used to deliver positive (systolic) and negative (diastolic) actuating forces to the ventricular surface (31).
Several patents with an aim of compressing part of the heart for cardiac assist have emerged. Heilman et al. created a ventricle compressing device consisting of multiple plates spaced around the side and apex of the ventricle that are actuated by a single linking band to cause ventricular compression (U.S. Patent No. 4,925,443) (32). This device compresses the ventricle from one or more sides in synchronization with the natural contraction of the ventricle and is completely implantable in the body of the patient and surroundings of the ventricles. Wilk has also created a compressive device that is fitted around the intra-pericardial space around the bottom portion of the heart (U.S. Patent No. 7,060,021 B1) (33). This device works by inserting a tensile membrane into patient for compression and closes off the lower portion of both ventricles. This device is suitable for emergency situations.
Other ECCDs have drawn inspiration from the concept of manual cardiac compression conducted during cardiopulmonary resuscitation (CPR). Finger-like devices that can “massage” a congestive heart failure patient’s heart to augment blood displacement have been developed. Shahinpoor has patented an implantable, multi-fingered robotic device controlled by a base platform (34). The fingers are attached to a central stem so when the stem cable is pulled, the attaching fingers curl and squeeze the heart simulating a clenching motion in systole. During diastole, the device quickly releases the clench allowing the heart to expand freely. In an alternate version of the device, assemblies of electro-active polymer fingers or patches are sutured to the myocardium and are directly and electrically flexed to squeeze the ventricles. In yet another embodiment of the device, an assembly of bladder-like fingers is inflated by hydrogen gas from an electrically controlled metal-hydride actuator to create compression of the ventricles.
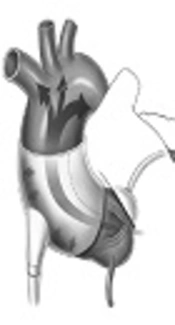
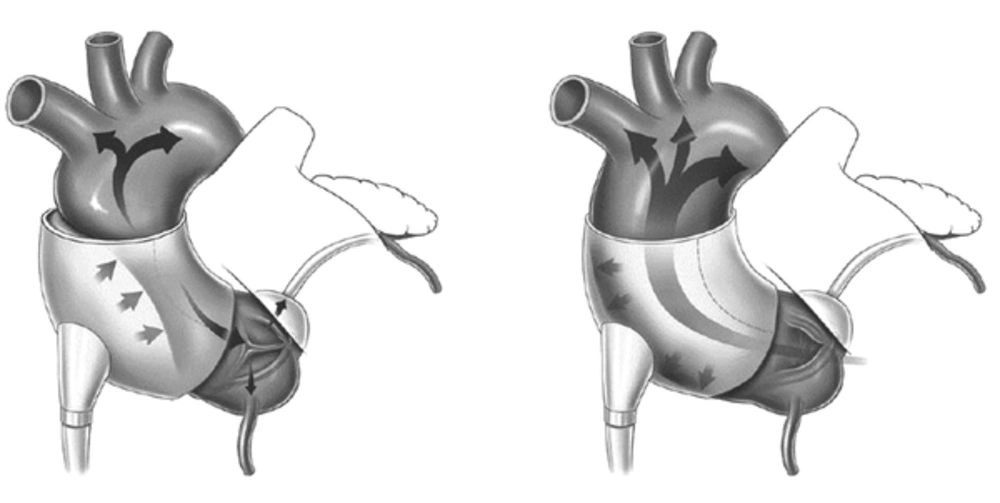
A device currently undergoing clinical trials is the C-Pulse Cuff extra-aortic counter-pulsation. Implantable through a rib or sternum minimally-invasive surgical procedure, the balloon cuff is wrapped around the ascending aorta and is placed right after the aortic valve (Figure 1). The device is synchronized with the patient’s ECG and inflates immediately after the closing of the aortic valve at the end of systole. This provides the circulatory system with additional cardiac output delivering more oxygenated blood downstream of the ascending aorta to areas such as the coronary arteries and vital organs. During systole, the cuff deflates and allows a reduction in afterload, reducing stress on the recovering left ventricle. The device targets patients with Class III and Class IV heart failure and aims to improve the quality of life and cardiac function.
3. Devices Drawing Energy from the Body
As an alternative to bulky external power supplies and percutaneous leads, some have explored alternative power sources to power a VAD such as utilizing energy from skeletal muscle. Several inventions and proposed surgical procedures have emerged intending to passively or actively involve skeletal muscle as part of an artificial apparatus for cardiac assist. Under normal physiologic conditions for adults at rest, cardiac output power between 1 to 2 watts is required to pump blood and ideally VADs should reliably deliver substantial power for up to years without recharging; thus internal energy sources are required to generate the equivalent energy for sustained continuous operation of these internal devices.
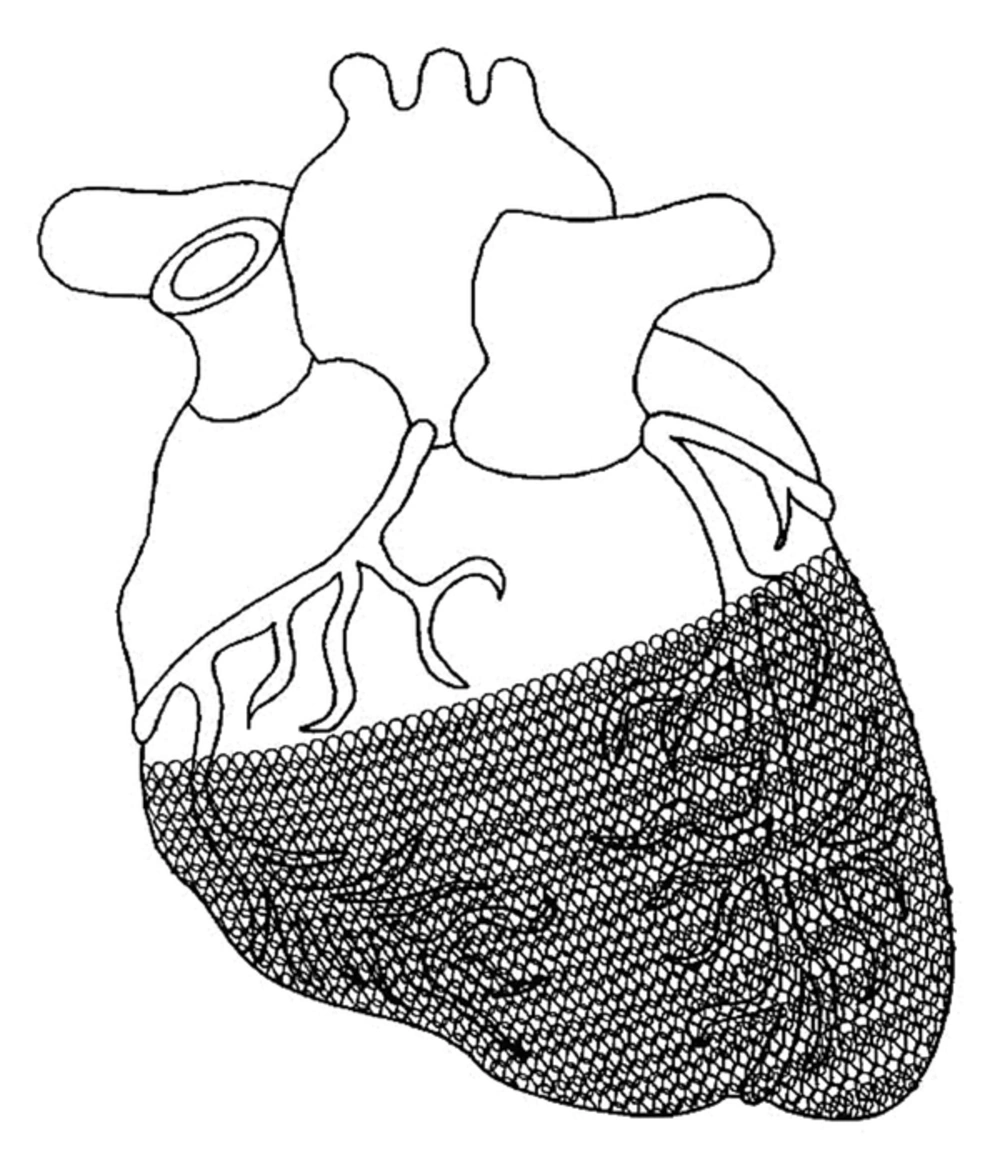
A passive support device previously marketed in Europe was the CorCap cardiac support device developed by acorn cardiovascular (Figure 2). The stocking-like polyester mesh is implanted via minimally invasive surgery to wrap around both ventricles to reverse the enlargement of ventricles (cardiomyopathy dilation) which is common in heart failure patients. The device provides end-diastolic support, reduces myocardium wall stress, and promotes myocardial recovery in heart failure patients. The device was approved for the European market and completed the first US clinical trials in 2005 with several follow-ups in hopes of FDA approval (35).
Badylak et al. developed an assist device connected between two points before and after the renal junction along the descending aorta (U.S. Patent No. 5,007,927) (36). The device consists of a flexible pouch chamber partially covered with rectus abdominis muscle. The inlet and outlet conduits connect the skeletal muscle device with the aorta. The rectus abdominis is translocated from its normal position, wrapped around a flexible pouch and sutured in place. The pulse generator receives electrical signals indicative of systole and diastole through sensor leads, generating a series of electrical pulses that stimulate contraction of relocated muscle tissue. The pulse generator also controls an electrically activated valve placed in the blood inlet conduit.
Chiu proposed a co-pulsation and counter-pulsation cardiac assist device, where the co-pulsation is achieved with an extra-cardiac ventricular cup for compressing the heart during systole and the counter pulsation is achieved via a jacket surrounding the aorta including a fluid expansible balloon for compressing a portion of the aorta during diastole (U.S. Patent No. 5,429,584) (37). Bellows are activated by the latissimus dorsi muscles for alternating the supply of fluid pressure to the co-pulsation and counter pulsation parts of the device. Sensors for the heart rate and electrodes for producing an electric pulse to contract selected muscles in response to the detected heart rate signals are implemented to produce the required alternating fluid flow.
Another invention offered by Campbell design is a method of making a left ventricular assist pump using muscle wrapped around a mandrel to form a muscle pouch, the open end of which is sewn to a circular sheet of patch material having connections to one end of each pair of vascular grafts, or alternatively to one end of a single vascular graft (U.S. Pat. No. 5,647,380) (38). The second end of these vascular grafts are used to connect the LVAD to the aorta. In other embodiment, an additional strip of latissimus dorsi or other appropriate muscle or a mechanical clamping device may be used to synchronously compress the aorta between the ends of the vascular grafts anastomosed to the aorta.
4. Results
4.1. Medical Considerations for ECCDs
Due to the configuration of current ECCDs, a major surgery such as thoracotomy or sternotomy is required for the implantation. Their use in earlier stage patients is thus restricted and it would only be beneficial to use the ECCDs for long-term application due to the invasiveness. Large device and complex mechanism will make the surgical procedure as challenging as the intracorporeal fixation. The size and weight of the device is a critical issue in smaller patients.
Other significant risks for the direct compression devices are the adhesion and tissue ingrowth. Cardiac recovery has been reported after mechanical assistance by VADs allowing the removal of the device. If this remodeling process would be expected, besides the invasive surgery required to withdraw the ECCD, device removal could result in substantial damage to the cardiac or vessel muscles due to tissue adhesion or ingrowth. Another situation in which a device removal is necessary is body rejection. Despite elimination or minimization of the direct blood contact in the ECCDs, the adjacency between artificial surfaces and internal organs is not avoidable. Further investigation on potential immune system activation due to the ECCDs is necessary before they are proven beneficial or capable of being alternatives to current VADs.
4.2. Engineering ECCDs
Devices incorporating fewer components such as Rubin and C-Pulse Cuff devices are favored from a reliability perspective and have a more direct route to successful application given the supporting technologies available. Both the Rubin and C-Pulse Cuff utilize external pneumatic pumping for power sources, which have successfully been implemented in VADs and other existing devices such as IABP and TAHs [citations: BerlinHeart, Pulsatile VADs, IABP]. Therefore, they can be readily engineered. With further research and clinical trials proving a 1) successful surgical procedure, 2) minimal material rejection inside the abdomen and 3) non-infection of transcutaneous leads, these devices would then be able to provide alternative therapies to VADs.
Engineering the Heilman, Wilk, Shahinpoor and Chandraseker devices to a level of successful operation within the body is less optimistic. The primary problem with these mechanically complex devices is the number of moving parts involved in operation that result in high probabilities of post-implantation mechanical breakdown. The malfunction of even a single component during the life of operation would require a surgery for repairmen. In addition, xenograft rejection, continual lubrication and heating are all problems hindering previous devices from sustainable continuous operation inside the abdomen. Finally, the contact points on the ventricle with some of these initial designs imply a problem with muscle damage after repetitive motions – a redesign with more delicate and dispersed force transfer to the ventricle is necessary; pneumatic filling mechanisms of Rubin and C-Pulse offer a superior solution than mechanical components.
One last hurdle required for both pneumatically and mechanically-based machines is the development of control systems to synchronize with the heartbeat. Customized EEG detection and control algorithms will need to be developed for each type of system, a difficulty experienced by VAD devices as well. From the authors’ experience, controlling and synchronizing with changes in heartbeat is more readily achieved by mechanical devices than pneumatically powered devices, which has a volume-dependent lag between the actuation and actual effect due to the compliance of the gas.
Inventions such as Badylak, Chiu, and Campbell devices offer an alternative method of cardiac assist that may circumscribe the need for customized and complex control systems, but also offer the most difficult engineering challenges. Initially, the question of whether such muscles can consistently and continuously offer the amount of energy necessary to assist the heart requires further research.
Utilization of intracorporeal power source, e.g. muscle power, is a major appeal in some ECCD inventions. From the medical point of view, using such a power source not only eliminates the transcutaneous catheter for conventional extracorporeal power supply that causes a high infection rate but also significantly increases mobility and thus improves patient’s quality of life by obviation of a large power console.
While controlling muscle tissue may pose a less engineering challenge than controlling mechanical or pneumatic components inside the body, healthy maintenance and sustainable operation is a question for both doctors and engineers with these devices. Badylak and Campbell devices are based on the challenging surgical relocation of healthy muscle tissue with blood circulation intact. Insufficient blood supply and muscle deterioration are highly probable with relocated muscles as reported with studies from and applied to these devices. Finally, the operation of these devices require blood contact with one-way valves which may bring challenges in thrombogenesis familiar with the first and second generation of VADs such as NovaCor.
5. Conclusions
An important question to ask in the cardiovascular disease arena is whether VAD can replace heart transplantation as a viable alternative and be even more beneficial to patients? It is necessary to find an alternative to heart transplantation quickly but current VADs cannot meet the equivalent reliability and longevity advantages of heart transplantation. Alternative solutions are ECCDs; those have the potential to replace heart transplantation and can revolutionize cardiovascular disease therapy.
History has shown that innovative thinking is more effective than pure advancement in technology and the simplest devices are often the most beneficial. Mechanical assist devices for the heart are a promising method for therapy but consist of advantages and disadvantages extensively covered by researchers. In order to move towards better device designs, there should be a focus on addressing current shortcomings by using a combination of advanced technologies as well as innovative thinking.
Advanced technology aims to find delicate and powerful engines. Considering the complications of today’s devices, many are impaired by fundamental design faults and not by motor size or power. The main factor that causes complications and reduces device life is direct contact to blood and the problem persists despite further advancements in technology. One main problem of cutting-edge technology is the high costs associated with the device, resulting in more than 90% of CHF patients not being able to afford it. Furthermore, the lack of post-operative care in developing countries exacerbates this problem.
If we accept the cardiac assist device as an appropriate alternative to transplantation, the problem of moving in the optimal direction as technologically and cost-effectively meeting the demand needs to be addressed. The following four guidelines are proposed to help in choosing the best design:
1. The less-blood-contact foreign surfaces due to less complications and longer device survival time.
2. The second guideline is related to device energy. Advancements in LVADs and batteries have allowed small batteries to supply devices with much lower energy requirements. However, the need for extracorporeal source of energy persists, leading to further burden and higher risks for the patient. An intracorporeal energy system would be ideal for cardiac assist devices.
3. The third guideline is to reduce percutaneous requirements of devices including catheter for fluid, blood or gas, controller signal wire and power cable. Infection is prevalent among LVAD patients and is mainly attributed percutaneous wiring and other parts to extracorporeal devices. Technology advancements should focus on reducing percutaneous leads towards fully wireless systems similar to today’s pacemakers.
4. The fourth guideline is to aim for smaller devices with simple and replaceable parts because more mechanical parts means higher breakdown risks and results in shortened device lifespans.
With consideration to the above guidelines, the ideal device will have no contact to blood and extracorporeal surfaces, lower dependency on extracorporeal sources of energy and is as simple as possible. While seemingly simple goals still require a large amount of research and development to achieve such a device.
Some ECCDs outlined above, despite being at a concept stage, match the guidelines for an ideal device better than current VAD technologies. Because of more research and advances in fields such as LVADs, and because of small size and only one moving part, impeller-based pumps have become a more popular therapy. Nevertheless, the inherent benefit of ECCDs not requiring contact to the blood to avoid complication has a promising future as an alternative. A device, ECCD or VAD, which can utilize both advanced technology and innovative thinking to achieve the ideal device, should be congratulated. We think, to design a new device, we should come back, think and see again. Simple devices with high technological material and intracorporeal source of energy will be an ideal design.