1. Background
From 2015 to 2018, about 121 million people worldwide have been diagnosed with diseases related to cardiac dysfunction (1). In Iran, the prevalence of heart diseases is estimated at about 10%, which is also increasing (2).
Recently, the relationship between thyroid hormone levels and cardiac complications has been considered (3, 4). The prevalence of thyroid diseases in the adult female population is between 9 and 15 percent, although this rate is lower in men (5, 6).
Thyroid dysfunction increases the risk of cardiovascular disease (3, 5). Thyroid hormone receptors are abundant in the myocardium; thus, the heart is very sensitive to changes in thyroid hormone (7). Disorders of thyroid hormones are associated with myocardial infarction, decreased cardiac output, altered heart rate, increased systemic vascular resistance, cardiomyocyte atrophy, endothelial dysfunction, and increased prevalence of atherosclerosis and heart failure (8). It has been observed that subclinical hypothyroidism is associated with left ventricular systolic and diastolic dysfunction (9, 10). Changes in thyroid hormone levels increase the hemodynamic load of the heart, which increases the heart rate and ultimately the size of the heart (11).
Thyroid hormones affect the action potential time and repolarization currents of cardiac myocytes (12). The findings show that the three hormones of TSH, T4, and T3 affect the heart and peripheral arteries, which leads to a decrease in systemic vascular resistance (SVR), and an increase in resting heart rate, left ventricular muscle contraction, and blood volume. In fact, thyroid hormones reduce resistance in peripheral arteries by directly affecting vascular smooth muscle (VSM) cell and reducing mean arterial pressure, thereby increasing the cardiac output pressure through reducing arterial resistance (13, 14). Endothelial-dependent vasodilation disorder has been reported as a result of nitric oxide reduction in subclinical hypothyroidism (15). The role of these hormones in heart failure has been identified, but limited studies have examined the systolic and diastolic ventricular disorders in detail (6, 9, 10, 12).
Given the roles of thyroid hormones in heart function and metabolism, assessing the levels of these hormones in patients with cardiac risk factors can be important.
2. Objectives
The present study aimed to assess the relationship between normal serum level of thyroid hormones and left ventricular systolic and diastolic dysfunction.
3. Methods
3.1. Study Design
In this retrospective cross sectional descriptive study, 391 patients, were selected. They were referred to the Ragaie cardiovascular research center, Tehran, Iran from 2019 to 2021. After extracting echocardiographic and laboratory data from the clinical records, the parameters affecting the left ventricular systolic and diastolic dysfunction were analyzed using statistical software. Dividing patients into normal, mild, moderate and severe in the measured parameters was based on ASE 2016 guideline for cardiac chamber quantification by echocardiography.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria included complete patient satisfaction, no symptoms of hyperthyroidism or hypothyroidism, and no history of treatment with thyroid drugs. Exclusion criteria included, distortion of clinical records, patients with specific diseases such as cancer, liver disorder, renal impairment, chronic infection, hematuria, heart failure, febrile illness, severe hyperglycemia, severe hypertension, and history of consumption of drugs that affect the thyroid test such as glucocorticoids, oral contraceptives, and nonsteroidal anti-inflammatory drugs.
3.3. Data Collection
Demographic and general information, including age and gender, were extracted from the clinical records of studied cases. Information about cardiac dysfunction was extracted from the clinical records of patients with the help of echocardiographic findings. Laboratory information such as TSH, T4, and T3 levels was also extracted from the patients' files. In case of defects in the file, the defects were eliminated through telephone calls. It should be noted that in this study, for all patients, the information about TSH was firstly recorded, and only in case of disorder of this hormone, information about other hormones and antibodies was recorded. Subclinical hypothyroidism was specified as normal levels of T4 and elevated levels of TSH, and clinical hypothyroidism was considered as reduced levels of T4 and increased levels of TSH. Also, hyperthyroidism was specified as increased T4 and reduced TSH levels, and subclinical hyperthyroidism was considered as normal T4 and reduced TSH levels. Normal serum TSH levels were considered to be between 0.4 and 4.2 mlU/L (16). Also, the normal level of total T4 was considered to be 4.4 to 11 mcg/dL in men and 4.8 to 12 mcg/dL in women (2). The normal range of total T3 was considered as 106 to 129 ng/dL in men and 104.5 to 140 ng/dL in women. In order to measure the laboratory variables, 8 cc blood samples had been taken from the subjects and were kept at -80°C. TSH, T4, and T3 levels were measured using the chemiluminescence method.
3.4. Statistical Analysis
After collecting the data by the checklist, SPSS software version 25 (IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) was used to analyze the data and compare the studied groups. Data are expressed as absolute frequency and percentages for qualitative variables. Quantitative variables are described as mean ± standard deviation (SD), depending on the variable distribution. Group comparisons were analyzed using the student t-test for continuous variables, and chi-square test or Fisher exact test for categorical variables. Because many comparisons were performed, P < 0.05 was considered as a cutoff value for statistical significance.
4. Results
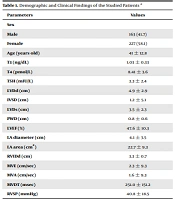
The patients’ mean age was 41 ± 12.8 years. 227 (58.1%) patients were female. Table 1 shows the demographic and clinical findings in the studied patients.
| Parameters | Values |
|---|---|
| Sex | |
| Male | 163 (41.7) |
| Female | 227 (58.1) |
| Age (years old) | 41 ± 12.8 |
| T3 (ng/dL) | 1.03 ± 0.33 |
| T4 (pmol/L) | 8.41 ± 3.6 |
| TSH (mIU/L) | 3.3 ± 2.4 |
| LVDd (cm) | 4.9 ± 2.9 |
| IVSD (cm) | 1.2 ± 5.1 |
| LVDs (cm) | 3.5 ± 2.3 |
| PWD (cm) | 0.8 ± 0.6 |
| LVEF (%) | 47.6 ± 10.3 |
| LA diameter (cm) | 4.1 ± 3.5 |
| LA area (cm2) | 22.7 ± 9.3 |
| RVIDd (cm) | 3.3 ± 0.7 |
| MVE (cm/sec) | 2.3 ± 9.3 |
| MVA (cm/sec) | 1.6 ± 9.3 |
| MVDT (msec) | 251.0 ± 151.2 |
| RVSP (mmHg) | 40.8 ± 18.5 |
Abbreviations: T3, triiodothyronine; TSH, thyrotropin stimulating hormone; T4, free thyroxine; T3, total T3; LVDd, left ventricular dimension in diastole; LVDs, left ventricular dimension in systole; IVSD, inter ventricular septum thickness in diastole; PWD, posterior wall thickness in diastole; LVEF, left ventricular ejection fraction; LA diameter, left atrial diameter; LA area, left atrial area; RVIDd, right ventricular dimension in diastole; MVE, mitral valve E wave velocity; MVA, mitral valve A wave velocity; MVDT, mitral valve inflow deceleration time; RVSP, right ventricular systolic pressure.
aValues are expressed as No. (%) or mean ± SD.
Evaluation for the relationship between cardiac parameters and thyroid hormone levels showed that tricuspid regurgitation (TR) severity was significantly associated with different levels of TSH in the normal range (P-value = 0.02).
According to Table 2, the study of the relationship between the studied parameters using Spearman's rank correlation coefficient showed that the levels of thyroid hormones were significantly related to some parameters. LVEF was inversely correlated with TSH levels (r = -0.10, P = 0.04), and interventricular septum thickness in diastole (IVSD) was inversely associated with T4 levels (r = -0.11, P = 0.04). Also, echocardiographic findings showed that mitral valve inflow deceleration time (MVDT) was inversely related to T3 (r = -0.15, P = 0.04). Also, right ventricular internal dimension in diastole (RVIDd) was directly related to TSH and this relationship was at the borderline of significance (r = 0.10, P = 0.05). Considering the statistical analyses performed, no significant relationship was observed between the other functional parameters and thyroid hormone levels (P > 0.05).
| LVDd | IVSd | LVDs | PWD | LVEF | LA diameter | LA area | RVIDd | MVDT | RVSP | |
|---|---|---|---|---|---|---|---|---|---|---|
| T3 (ng/dL) | ||||||||||
| r | 0.01 | 0.01 | 0.05 | -0.04 | -0.02 | -0.07 | -0.05 | 0.09 | -0.15 | 0.02 |
| P-value | 0.78 | 0.82 | 0.32 | 0.407 | 0.69 | 0.18 | 0.35 | 0.09 | 0.04 | 0.69 |
| T3 (pmol/L) | ||||||||||
| r | -0.05 | -0.11 | 0.03 | -0.06 | -0.09 | -0.01 | -0.05 | -0.02 | -0.13 | 0.04 |
| P-value | 0.30 | 0.04 | 0.46 | 0.24 | 0.06 | 0.74 | 0.38 | 0.68 | 0.08 | 0.46 |
| TSH (mIU/L) | ||||||||||
| r | -0.06 | -0.01 | -0.06 | -0.06 | -0.10 | 0.10 | 0.08 | 0.10 | -0.02 | 0.10 |
| P-value | 0.18 | 0.75 | 0.21 | 0.22 | 0.04 | 0.06 | 0.13 | 0.05 | 0.76 | 0.11 |
Abbreviations: LVDd, left ventricular dimension in end diastole; LVDs, left ventricular dimension in systole; IVSD, inter ventricular septum thickness in diastole; PWD, posterior wall thickness at end diastole; LVEF, left ventricular ejection fraction; LA diameter, left atrial diameter; LA area, left atrial area; RVIDd, right ventricular internal dimension in diastole; MVDT, mitral valve inflow deceleration time, RVSP, right ventricular systolic pressure.
5. Discussion
In the present study, it was shown that TR and RVIDd were positively correlated with TSH levels, and LVEF was inversely correlated with TSH levels. These findings are consistent with the results of the previous studies in this field (15, 17, 18). A similar study by Nakova et al. showed that TSH levels were inversely related to LVEF, and demonstrated a negative correlation (17). This finding indicates the importance of TSH levels in ventricular function. Another similar finding showed that TSH affects other thyroid hormones by altering the metabolism-related molecular pathways, and changes in the levels of this hormone are directly related to systolic and diastolic disorders (4, 8, 18). Of course, some studies have not shown this relationship (8, 9), but the effects of TSH levels on cardiac output and the ventricular wall have been proven (4, 15, 17, 18). In a study by Erkan et al., it was observed that the posterior ventricular wall thickness and mean diameter of the left atrium were higher in the group of patients with subclinical hypothyroidism (18).
The present study showed that diastolic interventricular septum thickness (IVSD) was inversely associated with T4, and mitral valve inflow deceleration time (MVDT) was inversely correlated with T3, which was consistent with the results of similar studies (19-21). Recent studies by Favuzzi et al. showed that a decrease in T3 was significantly associated with the right ventricular mid cavity diameter (21). Regarding the reduction of T3 in subclinical hypothyroidism, it has been reported that thyroid hormones affect the end-diastolic volume of the left and right ventricular (22, 23). Mousavi Mehdiabadi et al. stated that low T3 levels were negatively and significantly associated with the left ventricular function (24). Also, Neves et al. reported that heart rate was positively associated with T3, diastolic blood pressure was positively associated with TSH, the end systolic and diastolic volume of the LV was inversely related to TSH, and the ejection fraction was nonlinearly related to T3. In addition, the left posterior ventricular wall thickness was inversely related to T4 (25).
Our results also show that T4 levels are an influential factor in IVSD. Other studies have confirmed the association of T4 with heart disorders (25, 26) but the association between T4 levels and IVSD has not yet been established. In a study by Chaker et al., it was observed that a long-term increase in T4 levels is associated with the risk of coronary heart disease in patients with thyroid disorders (27). In another study, it was reported that T4 is an important factor affecting different types of heart disorders and changes in the level of this hormone are associated with the risk of heart disorders (20).
Some researches have shown that T3 and T4 improve several pathophysiological mechanisms associated with heart failure, including diastolic dysfunction and extra cardiac abnormalities (28). T3, as a biologically active hormone, affects cell signaling pathways and increases blood volume by increasing erythropoietin production and red blood cell mass. In hyperthyroidism, these changes lead to an increase in cardiac output. On the other hand, in hypothyroidism, cardiovascular changes are completely reversed and cardiac output is reduced (7). In general, studies have demonstrated the importance of understanding the mechanisms and roles of thyroid hormones in heart function and metabolism (27, 29). Jonklaas et al. have reported that determining the type of hormone used is one of the challenges in the treatment of heart disorders, and evaluating the effectiveness of different thyroid hormones on the heart can help to better understand this issue (30). The present study also had several strengths: First, the study population was in a good range. Secondly, this is the first study in Iran that specifically addresses the relationship between thyroid hormone levels and heart disorders. Lastly, different methods of analysis were used to assess hormone levels in patients with heart disorders, and this shows that our conclusions can be consistent and reliable.
Certainly, further clinical studies should be conducted regarding the effect of thyroid hormone supplements and the drugs that affect thyroid hormones in patients with heart diseases, and the regulation of these hormones can be an important therapeutic target.
5.1. Limitations
One of the limitations of our research was the incompleteness of some clinical files, which was resolved by following up the patients through telephone calls. Certainly, there are many known and unknown factors that may affect the function of the heart in patients, including the type of nutrition, genetics and stress, but it is certainly not possible to study all of these factors in one study. Thus, more studies should be conducted in wider statistical communities. Another limitation was that in this study, all patients with different types of thyroid disorders were studied, although classification of patients based on specific thyroid diseases can be effective in obtaining better results.
5.2. Conclusions
Tricuspid regurgitation severity and end diastolic right ventricular dimension were positively correlated with TSH, and LVEF was inversely correlated with TSH levels. Also, diastolic interventricular septal thickness (IVSD) was inversely associated with T4, and mitral valve inflow deceleration time (MVDT) was inversely correlated with T3. Due to the high prevalence of cardiac disorders, evaluation for their association with thyroid hormone levels can help long-term planning for the prevention and treatment of these disorders.