1. Introduction
Hypertension is the leading cause of mortality and morbidity worldwide (1, 2). It is estimated that nearly half of all cardiovascular diseases are attributed to uncontrolled hypertension (3). Intraoperative hypertension in cardiovascular operations may result in bleeding that may be diffuse or related to suture lines, resulting in retained hematoma and occasionally re-exploration. Moreover, high blood pressure may result in acute aortic dissection, acute pulmonary edema, kidney injury, or intracranial hemorrhage (4).
2. Case Presentation
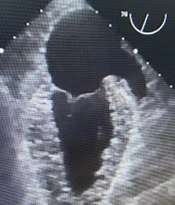
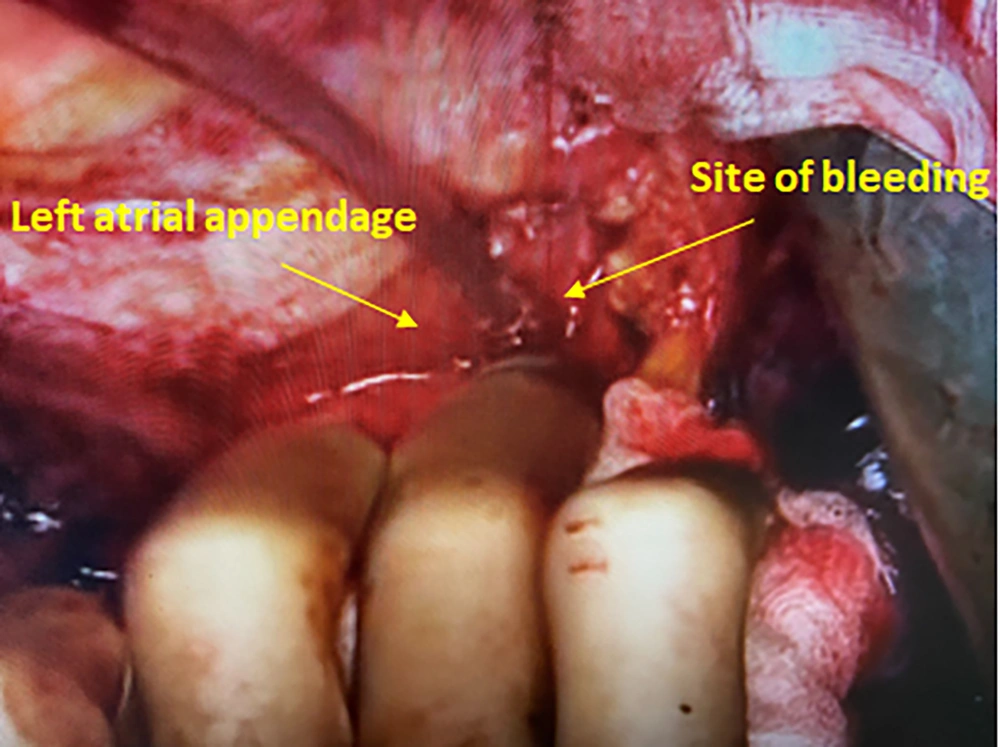
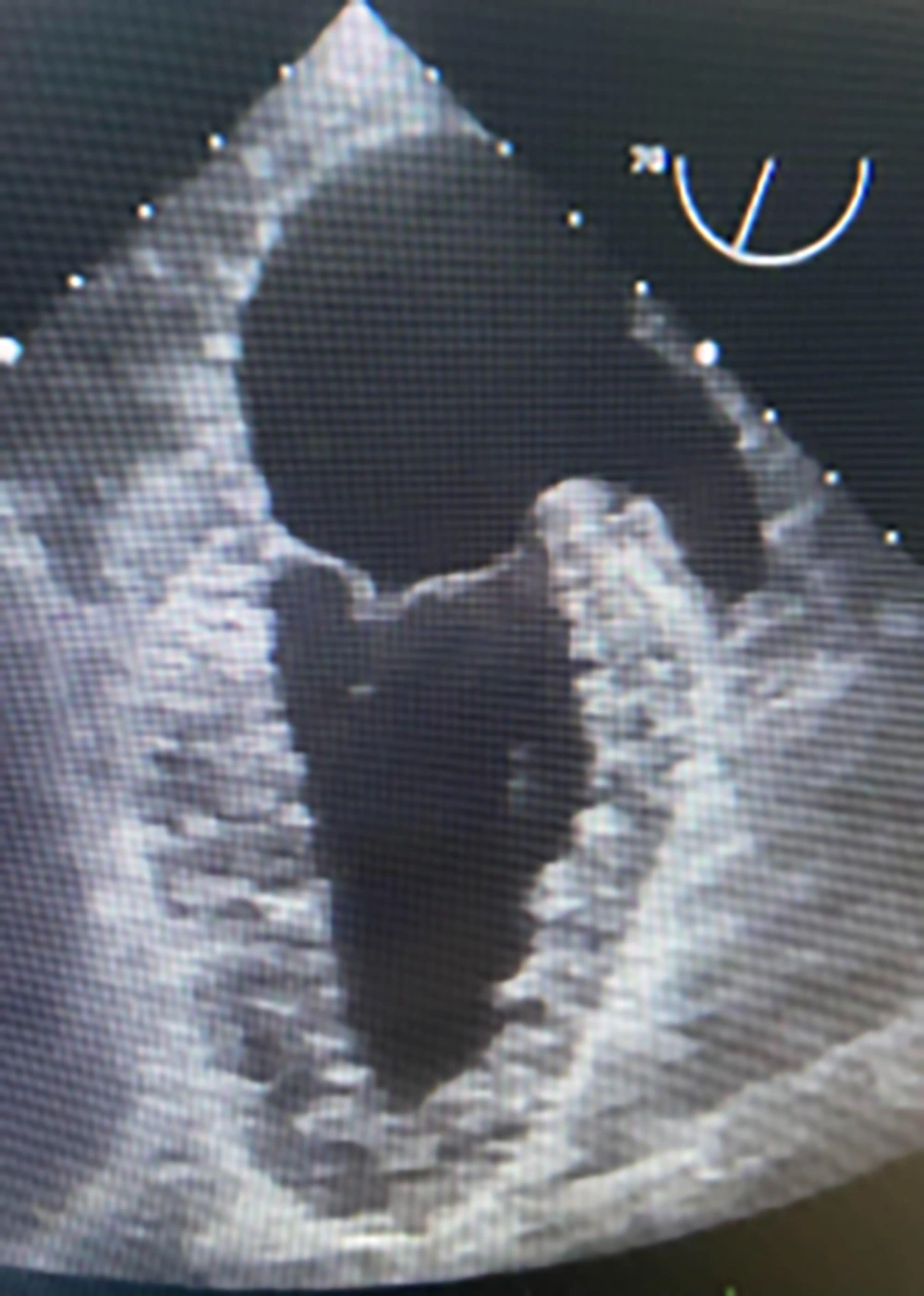
An 84-year-old woman with a long-standing history of hypertension and hyperlipidemia presented with stable angina and was found to have three vessels of coronary artery disease, including the left main. Preoperative echocardiography showed a left ventricular ejection fraction of 45% with inferonasal hypokinesis and moderate ischemic mitral regurgitation. She underwent on-pump coronary artery bypass grafting (CABG) x4 through a conventional approach utilizing the left internal mammary artery and saphenous vein grafts. She was easily weaned off cardiopulmonary bypass and decannulated. Following routine hemostasis and sternal closure, the patient became hypertensive with blood pressures as high as 165/90 mmHg. In an attempt to reduce blood pressure, the anesthesiologist inadvertently administered a bolus of epinephrine, resulting in severe tachycardia and hypertension with systolic blood pressure of nearly 240 mmHg. Shortly after, the patient developed marked bright red bleeding from the mediastinal drains. Upon re-exploration, bleeding was noted from behind the heart. After lifting the heart, a punctate site at the tip of the left atrial appendage (LAA) was found to be the source, which was repaired with a 4-0 prolene suture (Figure 1A). Distal and proximal anastomosis suture lines were hemostatic and free of bleeding. Intraoperative transesophageal echocardiography revealed near-normal wall motion without evidence of dissection in the heart chambers or the ascending aorta. (Figure 2) Although the patient’s postoperative course was complicated by delirium, she recovered completely and was discharged from the hospital on the sixth postoperative day.
3. Conclusions
To our knowledge, this is the first reported case of spontaneous left atrial appendage rupture during cardiac surgery. Previous research has described early and late tamponade following LAA percutaneous device closure (5, 6). From a surgical standpoint, the LAA auricle may be caught in the jaws of the aortic cross-clamp, causing iatrogenic injury (7). This complication is generally concerned with the limited view in minimally invasive operations as the cross-clamp may injure the LAA through the transverse sinus. To avoid this complication, it is crucial to visualize the tips of the cross-clamp during its application. Acknowledging the significance of this problem, we always raise the aorta under reduced bypass flow for cross-clamping and check the clamp site regularly. Regarding the mechanism of LAA injury, it can be assumed that a micro-perforation or crushing of the LAA had occurred. Following the procedure, the substantial increase in left ventricular pressure was most likely conveyed to the vulnerable left atrium via a regurgitant mitral valve. Left atrial dissection is another uncommon complication that might result in unexplained hematoma or hemorrhage following valve surgery. Its presence was excluded in our case by the general appearance of the appendage and via transesophageal echocardiography (Figure 1B). Technical considerations aside, this case illustrates the catastrophic consequences of medication errors in the operating room. Such errors may occur at any stage and may result from name confusion, prescription errors, and errors in dosage or route of administration (8).
Our patient and we were lucky that she did not suffer permanent damage. Had this complication occurred in the postoperative period, the chances of saving the patient without any neurological injury would have been remote.