1. Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with a worldwide prevalence of 2 - 3% (1, 2). Atrial fibrillation significantly increases the risk of morbidity and mortality, with a five-fold increased risk of stroke (3-5). For over fifty years, vitamin K antagonists (e.g., warfarin) were the sole option for oral anticoagulation in patients with AF. Some patients who declined warfarin therapy experienced rapid progression to chronic renal failure and acute renal injury due to excessive anticoagulation, supratherapeutic international normalized ratio (INR) values, and, in some cases, overt hematuria, a condition known as warfarin-related nephropathy (6, 7).
Since 2010, novel oral anticoagulants (NOACs) have been introduced and are now recommended over warfarin for most patients with AF. Although the side effects of these drugs, particularly regarding cerebral stroke and hemorrhagic episodes, have been thoroughly investigated in randomized controlled trials and observational studies, their impact on kidney function has been less studied.
Some studies suggest that NOACs may result in better kidney function compared to warfarin. Recent post-hoc analyses of studies such as RELY and ROCKET-AF indicated a more gradual decrease in estimated glomerular filtration rate (eGFR) among patients receiving dabigatran or rivaroxaban compared to those on warfarin (8, 9). This outcome may be due to the different pharmacological mechanisms of action. Warfarin inhibits vitamin K-dependent matrix proteins, including gamma-carboxy glutamic acid, which may lead to renal vessel calcifications and progressive nephropathy (10-12). In contrast, rivaroxaban inhibits factor Xa and thrombin, which may reduce inflammatory vasculopathy and theoretically protect kidney function (13, 14).
Few observational studies have compared NOACs with warfarin in terms of acute kidney injury (AKI). One study found a lower risk of AKI with dabigatran (15), while another study suggested that NOACs, especially rivaroxaban, may be associated with a lower risk of adverse kidney outcomes compared to warfarin (16). There is limited research on the renal implications of NOACs versus warfarin in AF patients with moderate renal insufficiency.
2. Objectives
This study aims to compare the effects of NOACs versus warfarin on renal function.
3. Methods
This study was designed as a prospective cohort study. The study population consisted of 99 patients recently diagnosed with non-valvular AF (< 1 year) and moderate chronic kidney disease (CKD), who were taking either warfarin or rivaroxaban for less than a year. It should be noted that the choice between these two treatments was based on the initial patient-doctor decision, and our role was limited to future follow-up.
All patients were referred to the Rajaie Cardiovascular Medical and Research Institute for follow-up between March 2018 and December 2021.
3.1. Inclusion Criteria
- Recently diagnosed with non-valvular AF for less than a year
- Age ≥ 18 years
- Glomerular filtration rate between 30 and 60 mL/min/1.73 m² (based on the MDRD formula)
Patients taking any well-known nephrotoxic medications were excluded from the study.
Upon arrival at our center, patients were either on warfarin or rivaroxaban, as previously prescribed by their primary cardiologist. A thorough history and document review were performed for each patient. Based on the inclusion criteria, cases were selected accordingly. All participants were informed about the study and signed the informed consent form.
Baseline creatinine, GFR, and CHA2DS2-VASc scores were calculated and documented based on initial laboratory data. This information, along with demographic and other clinical data, was recorded in a checklist. Follow-up visits at 6 and 12 months after the initial presentation were also evaluated and recorded. After data extraction, it was transferred to SPSS version 27 software and analyzed using the t-test and chi-square test.
4. Results
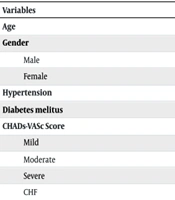
Ninety-nine patients with recently diagnosed non-valvular AF (< 1 year) and moderate renal insufficiency were on either warfarin or rivaroxaban treatment. The first group, consisting of 25 patients, was on warfarin, while the second group, comprising 74 patients, was on rivaroxaban. Gender distribution was even between the two groups (P-value: 0.6). Demographic data are shown in Table 1.
| Variables | Warfarin | Rivaroxaban | P-Value |
|---|---|---|---|
| Age | 72.92 ± 8.74 | 69.66 ± 8.87 | 0.114 |
| Gender | 0.6 | ||
| Male | 13 (52) | 40 (54.1) | |
| Female | 12 (48) | 34 (45.9) | |
| Hypertension | 17 (68) | 56 (76) | 0.45 |
| Diabetes melitus | 5 (20) | 17 (23) | 0.75 |
| CHADs-VASc Score | 0.56 | ||
| Mild | 0 (0) | 3 (4.1) | |
| Moderate | 2 (8) | 4 (5.4) | |
| Severe | 23 (92) | 67 (90.5) | |
| CHF | 5 (20) | 16 (21) | 0.48 |
a Values are expressed as mean ± SD or No. (%).
There was no significant difference in the CHADS-VASC score between the groups (P-value: 0.56).
Only 5 participants experienced major gastrointestinal bleeding, with no significant difference in distribution between the groups.
The baseline mean GFR was similar between the groups. The mean GFR in the rivaroxaban-treated group improved over 6 and 12 months, though this increase was not significant (P-value: 0.4). Conversely, GFR declined in the warfarin-treated group over 6 and 12 months, but this decline was not statistically significant (P-value: 0.913). All data are shown in Table 2.
| Variables Drug and Category | Mean ± SD |
|---|---|
| First GFR | |
| Rivaroxaban | 50.74 ± 7.57 |
| Warfarin | 47.15 ± 8.88 |
| GFR 6 months later | |
| Rivaroxaban | 51.83 ± 9.92 |
| Warfarin | 45.48 ± 10.51 |
| GFR 12 months later | |
| Rivaroxaban | 52.34 ± 8.65 |
| Warfarin | 45.46 ± 10.29 |
Abbreviation: GFR, glomerular filtration rate.
Notably, AKI occurred in 2 patients on rivaroxaban, characterized by a 30% decrease in GFR. Both cases were managed with dose-adjusted rivaroxaban. No patients progressed to end-stage renal disease (ESRD) or required dialysis during the study (Table 2).
In subgroup analysis, over the course of the study:
- In diabetic patients, GFR improved in the rivaroxaban-treated group and decreased in the warfarin group (P-value: 0.024). This change in GFR was not significant in nondiabetic patients (P-value: 0.2).
- In hypertensive patients, GFR improved in the rivaroxaban group and decreased in the warfarin group (P-value: 0.034). This change in GFR was not significant in patients without a history of hypertension (P-value: 0.7).
- In female patients, GFR improved in the rivaroxaban group and decreased in the warfarin group (P-value: 0.024). This change in GFR was not significant in male patients (P-value: 0.4).
- In high-risk patients for thrombus formation (high CHA2DS2-VASc score), GFR improved in the rivaroxaban-treated group and decreased in the warfarin group (P-value: 0.013). This change in GFR was not significant in low-risk patients (P-value: 0.84).
5. Discussion
Since the introduction of new oral anticoagulants (NOACs) in 2010, numerous studies have compared their effects with the conventional warfarin (4-7, 17). However, their impact on renal function has received less attention in previous research.
In our study, although GFR slightly decreased in patients treated with warfarin and slightly increased in those treated with rivaroxaban, these changes were not statistically significant. Despite the lack of significant GFR changes between the main groups, improvements in GFR were observed in the rivaroxaban-treated group compared to the warfarin group over time, specifically in diabetic, hypertensive, female, and high-risk patients with a high CHA2DS2-VASc score.
Previous studies, such as those by Brodsky et al., support our findings, indicating that warfarin-related nephropathy is more prevalent in patients with underlying CKD, with increased risk in individuals with additional comorbidities such as diabetes, hypertension, older age, and cardiac history (6).
The ROCKET AF and RE-LY trials demonstrated that in AF patients, rivaroxaban and dabigatran led to a slower decline in eGFR compared to warfarin (8, 9). Although extending the study duration and including more participants might yield significant results, our study did not find significant GFR changes between the groups over one year.
Chan et al. concluded that all three NOACs (apixaban, dabigatran, and rivaroxaban) are associated with a lower risk of AKI compared to warfarin among Asians with non-valvular AF in real-world settings (18). However, our study found two cases of AKI in the rivaroxaban-treated group and none in the warfarin-treated group, which may be attributed to ethnic differences.
Pastori et al. showed that patients on NOACs experienced a slower decline in renal function compared to those on warfarin, although this effect was partially diminished in patients with diabetes. Additionally, their study found that in patients aged >75 years, treatment with dabigatran and apixaban was associated with a slower rate of eGFR decline compared to warfarin (19). Conversely, Yao et al. reported that renal function decline is common among AF patients treated with NOACs (16). In our study, despite observing a slight GFR decline in the warfarin-treated group and a mild GFR increase in the rivaroxaban-treated group, no statistically significant differences were found between the groups overall. Significant GFR changes were only noted in specific subgroups, suggesting that more targeted treatment might benefit these populations.
It can be concluded that rivaroxaban may be a better option for female, diabetic, hypertensive, and high CHA2DS2-VASc score patients, while warfarin could be preferable for younger male patients. Further research on the renal effects of these two treatments is warranted.