1. Background
Heart transplantation is the treatment of choice for end-stage heart failure and is primarily performed in patients with severe ventricular systolic dysfunction due to end-stage cardiomyopathies that remain symptomatic despite optimal recommended treatment. International guidelines recommend heart transplantation for advanced heart failure, provided there are no contraindications (1).
Since the inception of heart transplantation for treating heart failure, early diagnosis of graft rejection has been crucial. Regular follow-up of patients aids in identifying complications such as acute graft rejection, vasculopathy of the coronary arteries of the transplanted heart, and chronic graft rejection (myocardial fibrosis), along with a reduction in heart contraction strength (2, 3).
According to International Society for Heart and Lung Transplantation (ISHLT) guidelines, there are four grades of cellular graft rejection based on histological findings (Table 1). The Quilty effect is defined as a focal, dense endocardial collection of lymphocytes that can extend into the adjacent myocardium (4).
| Grade | Histopathologic Findings |
|---|---|
| 0R, none | None |
| 1R, mild | Interstitial and/or perivascular infiltrate with up to 1 focus of myocyte damage |
| 2R, moderate | Two or more foci of infiltrate with associated myocyte damage |
| 3R, severe | Diffuse infiltrate with multifocal myocyte damage ± edema ± hemorrhage ± vasculitis |
Graft rejection is a major concern in the first year after transplantation, particularly within the first three months. Endomyocardial biopsy remains the gold standard for diagnosing acute or chronic graft rejection. However, tissue sampling is invasive and may be associated with complications, highlighting the need for non-invasive methods. Recently, non-invasive evaluation of coronary arteries, left ventricular function, and myocardial fibrosis has been explored using computed tomography and magnetic resonance imaging (3).
Currently, endomyocardial biopsy (EMB) is the most reliable method for detecting graft rejection, but it is associated with several issues, including patient discomfort, insufficient sample size, and potential errors in biopsy interpretation. While EMB is considered a safe procedure, it carries clinical risks (5). Therefore, finding a non-invasive method to detect graft rejection is beneficial. Several biochemical markers, such as creatine kinase (CK), troponin T and I, and interleukins, have been investigated, but results have been inconsistent (6, 7).
The CK-MB isoenzyme, present in myocardial tissue at relatively high concentrations, has long been used as a marker for diagnosing heart damage (8, 9). CK-MB levels may be useful in predicting the prognosis of coronary artery disease (CAD) as they are directly related to the extent of heart damage. Any form of heart damage, including that caused by trauma, surgery, inflammation, and ischemia, can elevate CK-MB levels in the serum (9).
2. Objectives
This study aims to determine the relationship between heart transplant rejection and serum levels of CK-MB in heart transplant patients.
3. Methods
In this prospective cohort study, 92 tissue samples were collected from adult patients who had undergone heart transplantation and were scheduled for routine EMB within the first three years of transplantation at Rajaie Cardiovascular Medical and Research Center in Tehran, Iran, during 2022. Serum samples for CK-MB levels were obtained immediately before the EMB procedure.
Exclusion criteria included the presence of any clinical and/or echocardiographic signs of graft dysfunction, coronary artery vasculopathy, systemic infection, malignancy, significant neurological issues, and active alcohol and/or tobacco use.
The EMB was performed in the catheterization laboratory via either the femoral or jugular approach under fluoroscopic guidance using standard cardiac bioptomes. Between 4 and 5 myocardial tissue samples were taken from the right ventricular side of the interventricular septum for pathological examination to assess acute cellular rejection.
Before and after the EMB procedures, a twelve-lead electrocardiogram and a standard transthoracic echocardiographic examination were conducted on all patients to detect conduction abnormalities, arrhythmias, and pericardial effusions.
The study was approved by the ethic committee of Iran university of medical sciences (ethic code: IR.IUMS.FMD.REC.1401.240) and a written informed consent was obtained from all the patients.
To determine serum CK-MB levels with high sensitivity, 5 cc of venous blood was drawn from the patients and sent to the hospital laboratory. Serum CK-MB levels were measured using the Audit kit, manufactured in Belgium, with a minimum positive limit set at 24 IU/L according to the kit brochure.
3.1. Statistical Analyses
All statistical analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to assess the normal distribution of variables. Quantitative variables were expressed as either median (interquartile range) or mean (standard deviation), while categorical variables were expressed as number (%), as appropriate.
Fisher's exact test, Kruskal-Wallis test, and Bonferroni's post hoc tests were used for comparisons and associations as appropriate. A P-value < 0.05 was considered significant.
4. Results
In this study, 56 samples (60.9%) were from male patients, and 36 samples (39.1%) were from female patients. The mean age (standard deviation) of the patients was 36.7 (9.59) years, with ages ranging from 19 to 64 years.
According to the specified cutoff, CK-MB serum levels were considered negative in 66 (71.7%) cases (biopsy samples), while 26 (28.3%) cases were considered positive for serum CK-MB levels.
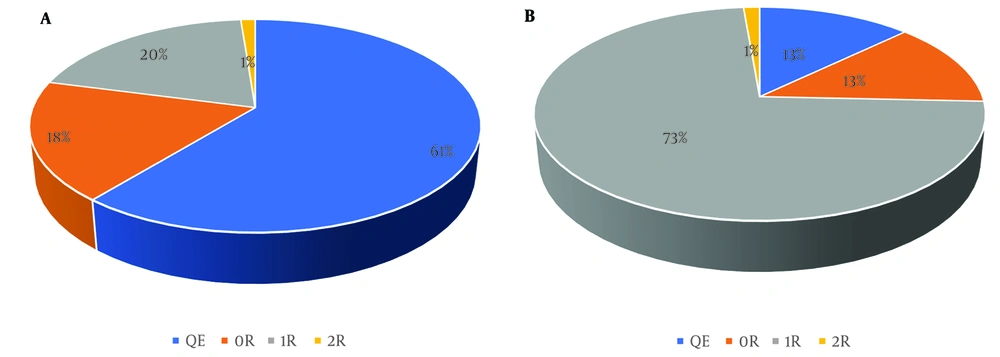
Figure 1A and B illustrate the association between grades of acute cellular rejection and concurrent quantitative serum levels of CK-MB. Most of the negative CK-MB results (< 24 U/L) were found in the QE group (61%), followed by the 0R group (18%).
A, relationship between graft rejection grade and qualitative evaluation of CK-MB in cases with negative CK-MB; B, relationship between graft rejection grade and qualitative evaluation of CK-MB in cases with positive CK-MB. Abbreviations: QE, Quilty effect; 0R, no rejection; 1R, grade 1 of rejection; 2R, grade 2 of rejection.
In contrast, the most common rejection grade among patients with positive serum CK-MB levels (≥ 24 U/L) was grade 1 (73%), with grade 0R being the next most common (13%).
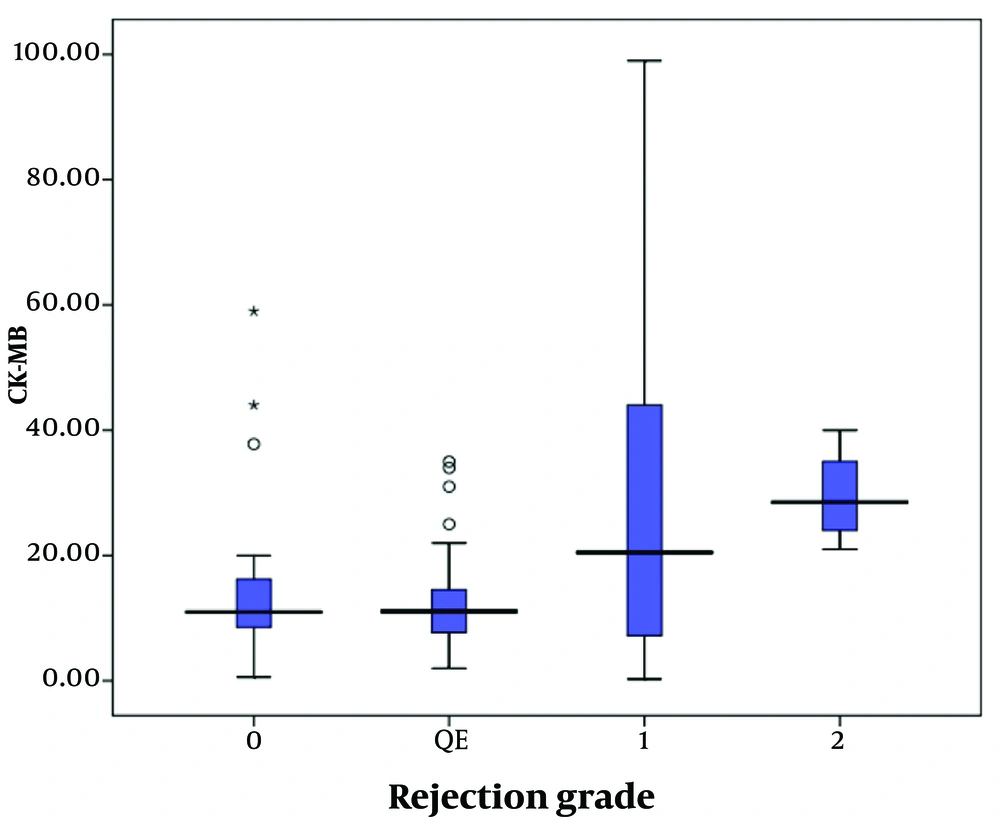
The quantitative measure of CK-MB serum levels across different grades of rejection is shown in Figure 2. The serum level of CK-MB was significantly higher in patients with higher degrees of rejection (P = 0.046). Post hoc multiple comparisons (using Bonferroni's post hoc test) revealed that the serum level of CK-MB in patients with QE was significantly lower than in those with grade 1R (P = 0.037) and grade 2R (P = 0.025). However, no significant differences were found in serum levels of CK-MB between 0R and 1R (P = 0.281), 0R and 2R (P = 0.071), 1R and 2R (P = 0.206), or between the QE group and grade 0R (P = 0.603).
The specificity and sensitivity of CK-MB for diagnosing any grade of rejection were 80% (95% CI = 51.9 - 95.6) and 58.8% (95% CI = 40.7 - 75.3), respectively.
5. Discussion
Measuring CK-MB in heart diseases is a non-invasive, low-cost, relatively sensitive, and straightforward method for determining cardiac injury and cardiovascular outcomes (9).
The present study demonstrates a statistically significant correlation between serum levels of CK-MB and the rejection grades of the transplant using qualitative measurement. In qualitative assessments, grade 0R mostly had a negative serum CK-MB level. Grade 1R cases had more frequent positive CK-MB results than grade 0R, and grade 2R had more positive results than grade 1R. However, in quantitative measurements, the mean CK-MB level was significantly lower in the QE group compared to the grade 1R and 2R groups. No significant statistical difference was observed between the different grades of graft rejection in terms of serum CK-MB levels.
The findings regarding the relationship between serum CK-MB and graft rejection grades are somewhat contradictory. Some studies suggest a relationship between serum CK-MB levels and graft rejection grades, while others have not reported such a relationship.
Similar to the present study, the laboratory study by Banon-Maneus et al. on rats with heart transplants indicated that CK is a valuable biochemical marker for predicting graft rejection. All rats with graft rejection had serum CK levels higher than 80 U/L. It was concluded that serum CK levels may predict moderate or severe graft rejection, and daily measurements could quickly detect moderate graft rejection (10).
In 2020, Jernryd et al. in Sweden studied myocardial damage biomarkers for early detection of graft dysfunction and found that patients with high serum CK-MB levels had a significantly higher risk of severe graft dysfunction (11).
Additionally, Balduini et al. in 2003 studied the relationship between biochemical markers (myoglobin, creatine kinase, troponin, serum amyloid, and CRP) and histological findings in heart transplant recipients. They found that serum CK-MB levels were significantly higher in patients with a positive biopsy for graft rejection compared to those with a negative biopsy (12).
In contrast, Moran et al. in 2002, studying children with heart transplants, did not find a significant relationship between the histological grade of graft rejection and serum CK-MB levels (7). Similarly, Ladowski et al. found that serum CK-MB levels did not have a predictive role for graft rejection or dysfunction (13).
5.1. Study Limitations
Although the careful selection of the study population to account for possible confounders was a strength of the present study, it is important to note that the specificity of tissue examination is not 100%, which may limit the conclusiveness of the results. This limitation could also explain the conflicting results found in similar studies.
Additional limitations of this study include the small sample size, the absence of grade 3 rejection cases, potential false-positive and/or false-negative results in CK-MB measurements, and the inconsistency in serum CK-MB levels. Furthermore, the study did not account for factors such as the duration of transplantation, the dose and serum levels of immunosuppressants, or donor-related variables.
5.2. Conclusions
In conclusion, while elevated serum levels of CK-MB in heart transplant patients may indicate graft rejection and could be useful for monitoring transplanted heart function, its sensitivity for diagnosing cardiac allograft rejection is very low. Therefore, endomyocardial biopsy and histopathological examination remain the gold standard for grading heart transplant rejection.