1. Background
Despite its crucial involvement in numerous cardiovascular diseases, right ventricular (RV) failure has historically received less attention than left ventricular (LV) dysfunction. Traditionally, the focus in cardiology has been predominantly on the LV, overshadowing the importance of the RV in various pathophysiological conditions (1). However, emerging evidence highlights the RV’s crucial prognostic significance, particularly in diseases such as pulmonary arterial hypertension (PAH), left heart failure, and even acute conditions like sepsis and acute respiratory distress syndrome (ARDS) (2-6). In these contexts, RV failure is associated with significant morbidity and mortality, especially in intensive care settings (4, 5).
Recent advances in echocardiographic imaging, including strain echocardiography, have provided new insights into RV function and its role in disease progression (1, 5, 7-10). Strain echocardiography, particularly speckle-tracking echocardiography, allows for the precise evaluation of RV myocardial function, assessing both systolic and diastolic phases by measuring free wall RV strain (5, 9, 10). This methodology has proved increasingly valuable in the clinical evaluation of RV dysfunction, providing a more sophisticated understanding of RV mechanics compared to traditional approaches.
Despite these advancements, the management of RV failure remains challenging. Pulmonary hypertension, for instance, leads to increased pulmonary artery pressure (PAP) and pulmonary vascular resistance (PVR), which can overwhelm the RV’s compensatory mechanisms, ultimately resulting in RV failure (11-13). This failure is often precipitated by conditions such as PAH secondary to left ventricular failure, pulmonary embolism, and post-cardiac surgery scenarios, where RV function is critically compromised (6).
Pharmacological interventions in RV failure have traditionally focused on inotropes, including dobutamine, milrinone, and levosimendan (5, 6). Milrinone, a phosphodiesterase 3 (PDE3) inhibitor, is frequently employed to enhance myocardial contractility and promote peripheral vasodilation in patients with ventricular dysfunction (11, 14-17). While its effects on LV function are well-documented, its impact on RV function, particularly in the context of RV strain, remains underexplored.
2. Objectives
Given the paucity of research specifically addressing the effects of milrinone on RV function, this observational echocardiographic study aims to investigate the changes in RV strain before and after milrinone administration in patients with RV failure. By focusing on the echocardiographic assessment of RV function, this study seeks to contribute to the growing body of evidence on the management of RV failure and to identify potential therapeutic benefits of milrinone in this challenging clinical scenario.
3. Methods
Following approval from the Ethics Committee of Shahid Rajaei Cardiovascular Medical and Research Center, Tehran (Approval ID: IR.RHC.REC.1402.058), this prospective observational study was conducted. Convenience sampling was used to recruit all eligible patients admitted to the cardiology or heart failure units at Shahid Rajaei Cardiovascular Medical and Research Center from December 2023 to June 2024.
3.1. Inclusion Criteria
- Patients over 18 years old with RV heart failure, defined as the S’ < 9.5, TAPSE < 16 and FAC < 35%.
3.2. Exclusion Criteria
- Prior use of inotropes or intravenous vasoactive agents before study enrollment.
- Patients with arrhythmias, including unstable rhythms such as frequent non-sustained ventricular tachycardia or poorly controlled atrial fibrillation (ventricular rate > 100 bpm).
- Hemodynamically unstable patients (e.g., systolic blood pressure < 80 mmHg, heart rate > 110 bpm).
- Patients with uncorrected primary valvular disease.
- Patients with left ventricular assist devices (LVAD), Impella devices, or intra-aortic balloon pumps.
- Patients contraindicated for milrinone.
- Patients who did not provide written informed consent for the use of their treatment data in the study.
- No exclusions were made based on race, gender, or ethnicity.
- Patients participating in other research projects during the study period were excluded.
3.3. Initial Evaluation
Before initiating treatment, demographic data (gender, age, body surface area), medical history (previous hospitalizations, hypertension, myocardial infarction, diabetes, hyperlipidemia, atrial fibrillation, chronic obstructive pulmonary disease), social history (smoking, opium use), and current medications (ACE inhibitors, ARBs, beta-blockers, calcium antagonists, diuretics, insulin, amiodarone, aspirin, digoxin, spironolactone, sacubitril, eplerenone) were recorded. Clinical findings included NYHA classification and the presence of peripheral edema. Echocardiographic data from speckle-tracking and strain echocardiography were also documented: LVEDD, LVESD, LVEDV, EF, E/E’, PAP, RV size, S’, TAPSE, four-chamber RVS, and free wall RVS.
3.4. Laboratory Data
Laboratory data included fasting blood sugar (FBS), hemoglobin (Hgb), creatinine (Cr), B-type natriuretic peptide (BNP), sodium (Na), potassium (K), urea, and blood urea nitrogen (BUN).
3.5. Intervention
Patients received milrinone with a bolus dose of 50 µg/kg administered over 10 minutes, followed by an infusion of 0.40 to 0.80 µg/kg/min for 20 minutes. Echocardiographic assessments were repeated 24 hours post-administration, including LVEDD, LVESD, LVEDV, EF, E/E’, PAP, RV size, S’, TAPSE, four-chamber RVS, and free wall RVS.
3.6. Echocardiographic Data Collection
Data were collected using a Vivid S60 Cardiac Ultrasound system (GE Healthcare) with a 3 to 8 MHz multiplane transthoracic echocardiography probe (GE Medical 6Tc or 6VT-D) at a frame rate of 50 - 70 frames per second, positioned in the mid-esophageal location. Right ventricular free wall speckle-tracking data in the mid-esophageal four-chamber longitudinal view were sampled during three consecutive heartbeats during apnea. Offline analysis was performed using TomTec Arena 2D strain echocardiography software. The endocardium was manually traced, and myocardial thickness was adjusted. Only segments confirmed by the software were analyzed using default settings for smoothing and strain compensation. Peak systolic strain, systolic strain rate (S wave, SR-S), and early diastolic filling strain rate (E wave, SR-E) were recorded for each of the three cardiac cycles and three lateral wall segments (apical, medial, basal). The average values for each segment and cycle were calculated.
3.7. Strain and Strain Rate Calculation
Myocardial strain was expressed as the fractional change (%) between end-diastole (L0) and end-systole (L), calculated as (L − L0)/L0 × 100. Strain rate (SR), representing the rate of deformation (contraction), was presented as 1/s. Negative values for strain and SR indicated myocardial contraction. TAPSE was measured in the four-chamber view using M-mode at the lateral tricuspid annulus (5, 18).
3.8. Clinical Evaluation
Clinical evaluation was based on the initial assessment data. Laboratory evaluations were conducted in the hospital's reference laboratory. Baseline disease and demographic data were obtained from patient medical records and self-reports
3.9. Statistical Analysis
Data were analyzed using SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0) and STATA (StataCorp. 2021. Stata: Release 17. Statistical Software). Descriptive statistics for quantitative variables were presented as mean (± standard deviation) and categorical variables as counts (percentages). Independent samples test or non-parametric equivalents were used to compare quantitative variables between genders. Pearson chi-Square test or Fisher's exact test was used for categorical variables. Logistic regression analysis evaluated categorical variables across study groups. Comparisons of echocardiographic assessments before and after milrinone administration were performed using paired-samples t-test or non-parametric equivalents. Simple linear regression and Pearson and Spearman correlations assessed the relationships between study variables.
4. Results
From the patients who visited our center during the specified period, only 25 met the criteria for inclusion in the study. Personal information and patient file numbers were stored for secondary review after their treatment course was completed. Upon completion of the study period, patient files were reviewed, and data were extracted from their medical records.
Among the 25 participants in this study, 15 (60%) were male, and 10 (40%) were female. The mean age (± standard deviation) of the participants was 51.54 years (± 15.847). The mean duration from the time of heart failure diagnosis to participation in the study was 11.29 years (± 8.111).
Of the 25 heart failure patients, 8 (32%) had idiopathic PH, 7 (28%) had ischemic cardiomyopathy (ICMP), 5 (20%) had dilated cardiomyopathy (DCM), 1 (4%) had restrictive cardiomyopathy (RCM), 1 (4%) had double outlet right ventricle (DORV), and 1 (4%) had myocarditis as the underlying cause of heart failure. Information about the underlying cause of RV heart failure was not available for two patients.
Additionally, 3 patients (12%) had a history of hypertension (HTN), 8 (32%) had a history of coronary artery disease (CAD), 4 (16%) had hyperlipidemia (HLP), 3 (12%) had chronic obstructive pulmonary disease (COPD), and 5 (20%) had diabetes mellitus (DM).
In terms of medication, 21 patients (84%) had a history of diuretic use, 11 (44%) had used spironolactone, 8 (32%) had used aspirin, 11 (44%) had used eplerenone, 7 (28%) had used beta-blockers, 7 (28%) had used sacubitril, 6 (24%) had used digoxin, 4 (16%) had used insulin, 2 (8%) had used amiodarone, and 1 (4%) had used ACE inhibitors. None of the patients had used ARBs or calcium channel blockers.
Regarding lifestyle habits, 5 patients (20%) were smokers, and 2 (8%) were opium users. Upon initial examination, 1 patient (4%) was classified as NYHA Class I, 16 (64%) as Class II, and 8 (32%) as Class III. Among the participants, 3 (12%) had pulmonary edema, and 8 (32%) had lower limb edema.
Electrocardiographic data revealed that 2 patients (8%) had atrial fibrillation (AF), 3 (12%) had right bundle branch block (RBBB), 5 (20%) had left bundle branch block (LBBB), 2 (8%) exhibited Q waves, and 4 (16%) had T-wave inversions. Additional demographic characteristics and initial laboratory results of the patients are provided in Table 1.
| Variables | Minimum - Maximum | Mean ± SD |
|---|---|---|
| Age (y) | 20 - 81 | 51.54 ± 15.84 |
| BSA (m²) | 1.47 - 16.00 | 2.37 ± 2.85 |
| Duration till Heart failure Diagnosed time (y) | 2 - 28 | 11.29 ± 8.11 |
| FBS (mg/dL) | 10.40 - 314.00 | 106.93 ± 53.19 |
| Hgb (g/dL) | 8.00 - 144.00 | 23.73 ± 36.10 |
| Cr (mg/dL) | 0.60 - 7.00 | 1.61 ± 1.30 |
| BNP (pg/mL) | 3.50 - 20315.00 | 3198.14 ± 5459.61 |
| Na (mEq/L) | 38.00 - 144.00 | 132.62 ± 20.80 |
| K (mEq/L) | 2.70 - 16.00 | 4.43 ± 2.46 |
| Urea (mg/dL) | 2.10 - 8.70 | 5.46 ± 1.70 |
Abbreviations: BSA, body surface area; FBS, fasting blood sugar; Hgb, hemoglobin; Cr, creatinine; BNP, B-type natriuretic peptide; Na, sodium; K, potassium; urea, blood urea nitrogen.
4.1. Age and Gender Comparisons
The mean age of male participants was 53.14 years (± 17.87), while that of female participants was 49.30 years (± 13.05), a difference that was not statistically significant (P > 0.05). The body surface area (BSA) averaged 1.95 (± 0.24) for males and 2.99 (± 4.56) for females, which was also not statistically significant (P > 0.05).
4.2. Laboratory Comparisons
The mean urea level was 6.00 (± 1.44) in males and 3.95 (± 1.59) in females, a difference that was statistically significant (P = 0.033). Other laboratory comparisons, including the duration since heart failure diagnosis, fasting blood sugar (FBS), hemoglobin (Hgb), creatinine (Cr), brain natriuretic peptide (BNP), and sodium (Na), did not reach statistical significance (P > 0.05) (Table 2).
| Variables | Gender | P-Value | |
|---|---|---|---|
| Male | Female | ||
| Age (y) | 53.14 ± 17.87 | 49.30 ± 13.05 | 0.570 a |
| BSA (m²) | 1.95 ± 0.24 | 2.99 ± 4.56 | 0.487 b |
| Duration till heart failure diagnosed time | 9.93 ± 8.10 | 13.20 ± 8.14 | 0.341 a |
| FBS (mg/dL) | 122.80 ± 60.04 | 83.14 ± 29.84 | 0.066 a |
| Hgb (g/dL) | 30.80 ± 45.78 | 13.13 ± 2.47 | 0.158 b |
| Cr (mg/dL) | 1.54 ± 0.79 | 1.71 ± 1.89 | 0.767 a |
| BNP (pg/mL) | 3617.13 ± 6157.36 | 2360.16 ± 4207.71 | 0.690 a |
| Na (mEq/L) | 131.20 ± 26.02 | 135.00 ± 6.96 | 0.675 a |
| K (mEq/L) | 3.94 ± 0.67 | 5.17 ± 3.80 | 0.229 a |
| Urea (mg/dL) | 6.00 ± 1.44 | 3.95 ± 1.59 | 0.033 a |
Abbreviations: BSA, body surface area; duration till heart failure diagnosed, years; FBS, fasting blood sugar; Hgb, hemoglobin; Cr, creatinine; BNP, B-type natriuretic peptide; Na, sodium; K, potassium; urea, blood urea nitrogen.
a Independent samples test.
b Mann-Whitney U test.
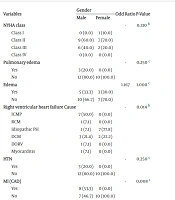
4.3. Clinical Examination Comparisons
There were no significant differences between males and females in NYHA class, pulmonary edema, and limb edema (P > 0.05). However, there was a significant difference in the causes of RV heart failure between genders (P = 0.014). Despite this, logistic regression analysis did not reveal significant differences for individual causes of RV heart failure between genders (P > 0.05).
In the male group, 8 patients (53.3%) had a history of CAD and prior myocardial infarction (MI), while none in the female group had CAD, a difference that was statistically significant (P = 0.008). No significant differences were found between genders in terms of HTN, HLP, COPD, and DM history (P > 0.05). Similarly, there were no significant gender differences in ECG findings and medication history, including AF, RBBB, LBBB, Q-wave, T-inversion, smoking, opium use, beta-blocker use, calcium channel blocker use, ACE inhibitor use, ARB use, digoxin use, sacubitril use, insulin use, spironolactone use, eplerenone use, aspirin use, amiodarone use, and diuretic use (P > 0.05) (Table 3).
| Variables | Gender | Odd Ratio | P-Value | |
|---|---|---|---|---|
| Male | Female | |||
| NYHA class | - | 0.310 b | ||
| Class I | 0 (0.0) | 1 (10.0) | ||
| Class II | 9 (60.0) | 7 (70.0) | ||
| Class III | 6 (40.0) | 2 (20.0) | ||
| Class IV | 0 (0.0) | 0 (0.0) | ||
| Pulmonary edema | - | 0.250 c | ||
| Yes | 3 (20.0) | 0 (0.0) | ||
| No | 12 (80.0) | 10 (100.0) | ||
| Edema | 1.167 | 1.000 c | ||
| Yes | 5 (33.3) | 3 (30.0) | ||
| No | 10 (66.7) | 7 (70.0) | ||
| Right ventricular heart failure Cause | - | 0.014 b | ||
| ICMP | 7 (50.0) | 0 (0.0) | ||
| RCM | 1 (7.1) | 0 (0.0) | ||
| Idiopathic PH | 1 (7.1) | 7 (77.8) | ||
| DCM | 3 (21.4) | 2 (22.2) | ||
| DORV | 1 (7.1) | 0 (0.0) | ||
| Myocarditis | 1 (7.1) | 0 (0.0) | ||
| HTN | - | 0.250 c | ||
| Yes | 3 (20.0) | 0 (0.0) | ||
| No | 12 (80.0) | 10 (100.0) | ||
| MI (CAD) | - | 0.008 c | ||
| Yes | 8 (53.3) | 0 (0.0) | ||
| No | 7 (46.7) | 10 (100.0) | ||
| HLP | - | 0.125 c | ||
| Yes | 4 (26.7) | 0 (0.0) | ||
| No | 11 (73.3) | 10 (100.0) | ||
| COPD | 0.286 | 0.543 c | ||
| Yes | 1 (6.7) | 2 (20.0) | ||
| No | 14 (93.3) | 8 (80.0) | ||
| DM | 1.000 | 1.000 c | ||
| Yes | 3 (20.0) | 2 (20.0) | ||
| No | 12 (80.0) | 8 (80.0) | ||
| AF rythem | - | 0.150 c | ||
| Yes | 0 (0.0) | 2 (20.0) | ||
| No | 15 (100.0) | 8 (80.0) | ||
| RBBB | 0.286 | 0.543 c | ||
| Yes | 1 (6.7) | 2 (20.0) | ||
| No | 14 (93.3) | 8 (80.0) | ||
| LBBB | 3.273 | 0.615 c | ||
| Yes | 4 (26.7) | 1 (10.0) | ||
| No | 11 (73.3) | 9 (90.0) | ||
| Q-wave | - | 0.500 c | ||
| Yes | 2 (13.3) | 0 (0.0) | ||
| No | 13 (86.7) | 10 (100.0) | ||
| T-inversion | - | 0.017 c | ||
| Yes | 0 (0.0) | 4 (40.0) | ||
| No | 15 (100.0) | 6 (60.0) | ||
| Smoke | 3.273 | 0.615 c | ||
| Yes | 4 (26.7) | 1 (10.0) | ||
| No | 11 (73.3) | 9 (90.0) | ||
| Opium | 0.643 | 1.000 c | ||
| Yes | 1 (6.7) | 1 (10.0) | ||
| No | 14 (93.3) | 9 (90.0) | ||
| B-blocker | 0.848 | 1.000 c | ||
| Yes | 4 (26.7) | 3 (30.0) | ||
| No | 11 (73.3) | 7 (70.0) | ||
| Ca-blocker | - | - | ||
| Yes | 0 (0.0) | 0 (0.0) | ||
| No | 15 (100.0) | 10 (100.0) | ||
| ACEIs | - | 1.000 c | ||
| Yes | 1 (6.7) | 0 (0.0) | ||
| No | 14 (93.3) | 10 (100.0) | ||
| Digoxine | 1.455 | 1.000 c | ||
| Yes | 4 (26.7) | 2 (20.0) | ||
| No | 11 (73.3) | 8 (80.0) | ||
| Sacubitril | 6.000 | 0.179 c | ||
| Yes | 6 (40.0) | 1 (10.0) | ||
| No | 9 (60.0) | 9 (90.0) | ||
| Insulin | - | 0.125 c | ||
| Yes | 4 (26.7) | 0 (0.0) | ||
| No | 11 (73.3) | 10 (100.0) | ||
| Spironollactone | 0.333 | 0.241 c | ||
| Yes | 5 (33.3) | 6 (60.0) | ||
| No | 10 (66.7) | 4 (40.0) | ||
| Eplernone | 1.313 | 1.000 c | ||
| Yes | 7 (46.7) | 4 (40.0) | ||
| No | 8 (53.3) | 6 (60.0) | ||
| ARBs | - | - | ||
| Yes | 0 (0.0) | 0 (0.0) | ||
| No | 15 (100.0) | 10 (100.0) | ||
| Aspirin | 7.875 | 0.088 c | ||
| Yes | 7 (46.7) | 1 (10.0) | ||
| No | 8 (53.3) | 9 (90.0) | ||
| Amiodarone | 0.643 | 1.000 c | ||
| Yes | 1 (6.7) | 1 (10.0) | ||
| No | 14 (93.3) | 9 (90.0) | ||
| Diuretics | 1.625 | 1.000 c | ||
| Yes | 13 (86.7) | 8 (80.0) | ||
| No | 2 (13.3) | 2 (20.0) | ||
Abbreviations: NYHA class, New York Heart Association functional classification; HTN, hypertension; MI (CAD), myocardial infarction (coronary artery disease); HLP, hyperlipidemia; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; AF rythem, atrial fibrillation rhythm; RBBB, right bundle branch block; LBBB, left bundle branch block; Ca-Blocker, calcium channel blockers; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ICMP, ischemic cardiomyopathy; RCM, restrictive cardiomyopathy; DCM, dilated cardiomyopathy; DORV, double outlet right ventricle.
a Values are expressed as No. (%).
b Pearson chi-square test.
c Fisher's exact test.
4.4. Echocardiographic Findings and Milrinone Administration
Comparing echocardiographic results before and after milrinone administration showed a mean EF of 27.80 (± 17.32) before and 29.78 (± 18.12) after administration, which was not statistically significant (P > 0.05). The mean E/E' was 17.64 (± 17.98) before and 17.86 (± 11.38) after administration, which was also not significant (P > 0.05). However, the mean PAP was significantly reduced from 57.16 (± 31.89) before to 39.86 (± 21.75) after milrinone administration (P < 0.001).
The mean RV size decreased significantly from 4.14 (± 0.93) before to 3.81 (± 0.83) after milrinone administration (P = 0.004). The mean S’ remained unchanged at 9.00 (± 3.02) before and after milrinone administration (P > 0.05). The mean TAPSE was 14.95 (± 3.41) before and 14.34 (± 2.88) after milrinone administration, showing no significant difference (P > 0.05). The mean four-chamber RV strain improved significantly from -8.75 (± 4.01) to -10.58 (± 4.66) (P = 0.025). The free wall RV strain also showed a significant improvement from -11.91 (± 4.74) to -14.76 (± 5.44) (P = 0.003). Other echocardiographic data are presented in Table 4.
| Variables | Before Milrinone | After Milrinone | P-Value |
|---|---|---|---|
| LVEDD (mm) | 6.03 ± 1.64 | - | - |
| LVESD (mm) | 4.90 ± 1.86 | - | - |
| LVEDV (mL) | 166.60 ± 113.50 | - | - |
| EF (%) | 27.80 ± 17.32 | 29.78 ± 18.12 | 0.066 b |
| E/E’ | 17.64 ± 17.98 | 17.86 ± 11.38 | 0.306 b |
| PAP (mmHg) | 57.16 ± 31.89 | 39.86 ± 21.75 | > 0.001 b |
| RV size | 4.14 ± 0.93 | 3.81 ± 0.83 | 0.004 c |
| S’ (cm/s) | 9.00 ± 3.02 | 9.00 ± 4.93 | 0.193 b |
| TAPSE (mm) | 14.95 ± 3.41 | 14.34 ± 2.88 | 0.250 b |
| Four chamber RVS | -8.75 ± 4.01 | -10.58 ± 4.66 | 0.025 b |
| Free wall RVS | -11.91 ± 4.74 | -14.76 ± 5.44 | 0.003 c |
Abbreviations: LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; EF, ejection fraction; E/E’, ratio of early mitral inflow velocity (E) to early diastolic mitral annular velocity (E’); PAP, pulmonary artery pressure; RV size, right ventricular size; S’, systolic excursion velocity of the tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; RVS, right ventricular strain.
a Values are expressed as mean ± SD.
b Wilcoxon signed-rank test.
c Paired-samples t-test.
4.5. Gender Comparisons of Echocardiographic Changes
The mean LVEDD before milrinone administration was 6.89 (± 1.30) in males and 4.72 (± 1.23) in females, a statistically significant difference (P = 0.001). The mean LVESD was 5.89 (± 1.41) in males and 3.38 (± 1.41) in females, which was also significant (P < 0.001). The mean LVEDV was 217.36 (± 104.07) in males and 87.67 (± 79.42) in females, a difference that was statistically significant (P = 0.005). The mean EF before milrinone administration was 19.00 (± 13.65) in males and 41.00 (± 13.70) in females, which was significant (P = 0.001).
The mean four-chamber RVS was -7.40 (± 2.55) in males and -10.78 (± 5.02) in females before milrinone administration, a statistically significant difference (P = 0.036). There were no significant differences between genders in E/E', PAP, RV size, S’, TAPSE, and free wall RVS before milrinone administration (P > 0.05). The mean EF after milrinone administration was 17.69 (± 13.33) in males and 45.50 (± 8.96) in females, a statistically significant difference (P < 0.001).
No significant gender differences were found in E/E', PAP, RV size, S’, TAPSE, four-chamber RVS, and free wall RVS after milrinone administration (P > 0.05). Post-administration LVEDD, LVESD, and LVEDV data were not available. Additionally, differences in echocardiographic parameters before and after milrinone administration, including EF, E/E', PAP, RV size, S’, TAPSE, four-chamber RVS, and free wall RVS, did not show significant gender differences (P > 0.05) (Table 5).
| Variables | Gender | P-Value | |
|---|---|---|---|
| Male | Female | ||
| LVEDD (mm) | |||
| Before milrinone | 6.89 ± 1.30 | 4.72 ± 1.23 | 0.001 b |
| After milrinone | - | - | - |
| Measured difference | - | - | - |
| LVESD (mm) | |||
| Before milrinone | 5.89 ± 1.41 | 3.38 ± 1.41 | < 0.001 b |
| After milrinone | - | - | - |
| Measured difference | - | - | - |
| LVEDV (mL) | |||
| Before milrinone | 217.36 ± 104.07 | 87.67 ± 79.42 | 0.005 b |
| After milrinone | - | - | - |
| Measured difference | - | - | - |
| EF (%) | |||
| Before milrinone | 19.00 ± 13.65 | 41.00 ± 13.70 | 0.001 b |
| After milrinone | 17.69 ± 13.33 | 45.50 ± 8.96 | < 0.001 b |
| Measured difference | 0.00 ± 0.00 | 4.50 ± 7.25 | 0.081 c |
| E/E’ | |||
| Before milrinone | 22.29 ± 22.73 | 11.61 ± 5.48 | 0.163 b |
| After milrinone | 20.51 ± 12.70 | 13.33 ± 7.46 | 0.193 b |
| Measured difference | -2.79 ± 27.53 | 3.31 ± 8.91 | 0.580 b |
| PAP (mmHg) | |||
| Before milrinone | 49.27 ± 23.08 | 69.00 ± 40.30 | 0.184 c |
| After milrinone | 35.92 ± 18.03 | 45.00 ± 25.93 | 0.333 b |
| Measured difference | -15.77 ± 19.25 | -24.00 ± 20.46 | 0.334 b |
| RV size | |||
| Before milrinone | 4.16 ± 1.03 | 4.27 ± 1.02 | 0.806 b |
| After milrinone | 3.83 ± 0.90 | 3.80 ± 0.80 | 0.935 b |
| Measured difference | -0.19 ± 0.50 | -0.54 ± 0.36 | 0.086 b |
| S’ (cm/s) | |||
| Before milrinone | 8.21 ± 2.55 | 10.10 ± 3.41 | 0.135 b |
| After milrinone | 9.54 ± 6.32 | 8.30 ± 2.31 | 0.563 b |
| Measured difference | 1.54 ± 6.60 | -1.80 ± 2.66 | 0.148 b |
| TAPSE (mm) | |||
| Before milrinone | 14.14 ± 3.08 | 16.10 ± 3.70 | 0.172 b |
| After milrinone | 13.92 ± 1.93 | 14.90 ± 3.84 | 0.476 c |
| Measured difference | -0.08 ± 2.56 | -1.20 ± 5.73 | 0.534 b |
| Four chamber RVS | |||
| Before milrinone | -7.40 ± 2.55 | -10.78 ± 5.02 | 0.036 b |
| After milrinone | -9.41 ± 3.39 | -12.33 ± 5.87 | 0.178 c |
| Measured difference | -2.01 ± 3.27 | -1.55 ± 3.10 | 0.726 b |
| Free wall RVS | |||
| Before milrinone | -11.04 ± 4.45 | -13.23 ± 5.11 | 0.267 b |
| After milrinone | -13.47 ± 4.70 | -16.71 ± 6.15 | 0.149 b |
| Measured difference | -2.43 ± 4.59 | -3.48 ± 4.02 | 0.564 b |
Abbreviations: LVEDD, left ventricular end-diastolic diameter; LVESD, Left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; EF, ejection fraction; E/E’, ratio of early mitral inflow velocity (E) to early diastolic mitral annular velocity (E’); PAP, pulmonary artery pressure; RV size, right ventricular size; S’, systolic excursion velocity of the tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; RVS, right ventricular strain.
a Values are expressed as mean ± SD.
b Independent samples test.
c Mann-Whitney U test.
4.6. Correlation Analyses
Changes in echocardiographic parameters before and after milrinone administration were calculated. The Kruskal-Wallis test revealed a significant association between beta-blocker use and changes in four-chamber RVS [X2 (1) = 7.61, P = 0.005] and free wall RVS [X2 (1) = 7.09, P = 0.007]. Other investigations regarding sex, clinical examinations (including NYHA class, edema, pulmonary edema), etiology of heart failure, history of HTN, MI (CAD), hyperlipidemia, COPD, and diabetes mellitus, EKG findings (including presence or absence of AF rhythm, RBBB, LBBB, Q wave, T inversion), history of smoking, opium use, and medications such as ACE inhibitors, digoxin, sacubitril, insulin, spironolactone, eplerenone, aspirin (ASA), Amiodarone, and Diuretics did not reach statistical significance (P > 0.05). Furthermore, the results of simple linear regression analysis and Pearson correlation for the relationship between age and duration of heart failure diagnosis in patients with differences in RVS before and after receiving Milrinone, as well as Spearman correlation analysis to investigate the relationship between BSA and differences in RVS before and after receiving Milrinone, did not reach statistical significance (P > 0.05).
5. Discussion
Our study demonstrated that the average urea levels were higher in the male group compared to the female group. Yano et al. previously highlighted that blood urea nitrogen (BUN) serves as a valuable marker for composite outcomes like all-cause mortality and heart failure (HF) readmission, independent of baseline renal function, and correlates with left atrial function in HF with preserved ejection fraction (HFpEF) patients (19). Ru et al. found that acute and chronic HF were associated with a 36% and 30% incidence of acute kidney injury (AKI), respectively, emphasizing the relationship between BUN levels and AKI occurrence in HF patients (20). Although our study did not find significant differences in creatinine (Cr) levels between genders, renal impairment remains a critical consideration in acute and chronic HF due to reduced renal perfusion and nephron damage, indicating disease progression and heightened mortality risk (21-24). Zhu et al. reported that an elevated BUN/Cr ratio predicts poor prognosis and increased all-cause mortality in AF patients, further suggesting worse disease outcomes in men compared to women (25-27).
We observed a notable disparity in the causes of RV heart failure between men and women in our study, although logistic regression analysis did not reach statistical significance. The global burden of disease study identified 17 etiologies for heart failure, highlighting ischemic heart disease as a prominent global burden, with varying incidences across different regions (28). Baldasseroni et al. reported the distribution of dilated cardiomyopathy, ischemic cardiomyopathy, hypertensive cardiomyopathy, and other causes without specific gender-related analysis (29). Quantifying the global burden of cardiomyopathy and congenital heart diseases remains challenging, particularly in developing countries where diagnostic limitations prevail (30, 31). Nevertheless, the modest sample size in our study may have influenced our findings.
We found a significant discrepancy in the prevalence of coronary artery disease (CAD) between male and female groups, with a higher prevalence in males. This observation aligns with existing literature attributing disparities to factors such as differential symptom recognition in women, delayed diagnosis, comorbidity burden at diagnosis, suboptimal risk factor management, and higher in-hospital mortality following CAD events compared to men (32-36).
Our study revealed a significant gender difference in the occurrence of T-wave inversion on electrocardiograms (ECGs), with a higher prevalence among women. This finding is consistent with Mieszczanska et al., who reported similar gender-related differences in ECG parameters following myocardial infarction (MI) (37). Although our study did not focus extensively on specific ECG leads for T-wave inversion, the meta-analysis by Bazoukis et al. corroborates adverse outcomes associated with T-wave inversion in certain ECG leads, which may explain our findings regarding CAD history among our patient cohort (38).
Regarding echocardiographic findings, initial assessments showed higher left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), and LVEDV in men compared to women. This contrasts with normal echocardiographic parameters, suggesting increased values beyond the upper limit for men (39). Conversely, initial evaluations indicated higher ejection fraction (EF) and four-chamber RVS in women, consistent with Larranaga-Moreira et al., who reported a higher prevalence of left ventricular EF < 50% in men presenting with syncope (40). Despite overall reduced EF and RVS in our study cohort compared to normal thresholds, our findings are limited by the absence of left ventricular global longitudinal strain (LVGLS) assessments (39).
Subsequent echocardiographic evaluations post-milrinone administration revealed significantly improved EF in the female group, consistent with prior research highlighting gender-based EF disparities (39, 40).
Evaluation of echocardiographic findings pre- and post-milrinone administration was the primary objective of our study. We observed a significant reduction in PAP post-milrinone administration, in line with findings from Wang et al. on the efficacy of intravenous and inhaled milrinone in PAH (41). Patel et al. similarly reported reduced mean and systolic pulmonary artery pressures following milrinone administration after cardiopulmonary bypass, although Fredholm et al. noted increased pressures post-milrinone administration following aortic valve replacement (5, 42). These discrepancies may stem from differing study designs and sample sizes but underscore milrinone's potential in preventing complications associated with RV failure (43).
Our findings further demonstrated that milrinone treatment reduced RV size and raised four-chamber and free wall RV strain, which is consistent with the results published by Fredholm et al. (5) and James et al. in infant populations (44).
Notably, a significant association was observed between prior β-blocker use and changes in four-chamber and free wall RV strain following milrinone administration. This suggests a synergistic effect between β-blockers and milrinone in enhancing RV strain improvement. Although the co-administration of inotropes and β-blockers in acute decompensated heart failure has been theoretically discussed, empirical evidence specifically assessing their combined effects remains scarce (16, 45).
Milrinone inhibits phosphodiesterase-3 (PDE-3), thereby increasing intracellular cyclic adenosine monophosphate (cAMP) levels, which enhances myocardial contractility by augmenting calcium entry into cardiac myocytes and facilitating vascular smooth muscle relaxation (16). Unlike β-agonists, which exert their effects via β1-receptor activation, milrinone may offer advantages in patients receiving β-adrenergic blockade, thereby potentially improving cardiac output, reducing pulmonary wedge pressure, and lowering myocardial oxygen consumption (46-48).
Despite efforts to mitigate biases, our study is limited by its small sample size, which reduces statistical power. Additionally, the inability to conduct secondary evaluations on some patients post-milrinone administration underscores the need for future studies addressing these shortcomings.
5.1. Conclusions
In conclusion, our study revealed a greater severity of heart failure associated with male gender within our study population. Furthermore, our findings underscored the efficacy of intravenous milrinone in enhancing right heart function and RV strain. Specifically, our results demonstrated that prior beta-blocker use was associated with more pronounced improvements in right heart function following milrinone administration compared to baseline measurements. These findings suggest a synergistic benefit of combining beta-blockers with milrinone in optimizing right heart performance among heart failure patients.